In the stem cells therapy, the induced pluripotent stem cells (iPSCs) which are directly derived from patients' somatic cells reprogramming to an embryonic-like state. Since iPSCs could be coaxed into the desired cell types that would already be genetically matched with the patient, the immune rejection and the ethical issues can be potentially circumvented.
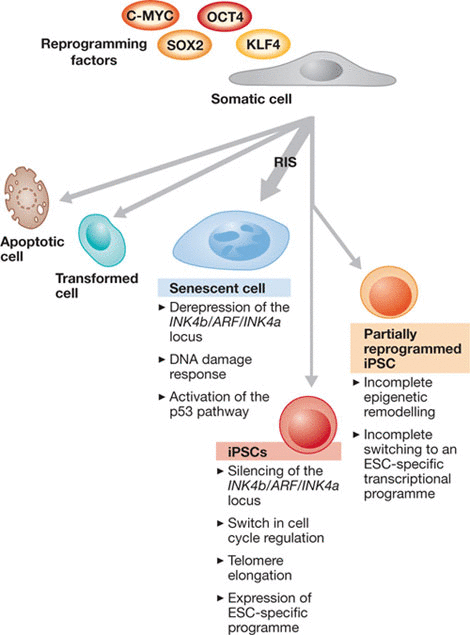
There are three areas of research and the influence they had on the generation of iPSCs. Firstly, the somatic cell nuclear transfer (SCNT) provided the possibility of differentiated cells retain the same genetic information as early embryonic cells; Then, the development of techniques allowed researchers to derive, culture, and study pluripotent cell lines; Lastly, the observation that transcription factors are key determinants of cell fate whose enforced expression can switch one mature cell type into another let iPSCs technology become true. By the introduction of a defined and limited set of transcription factors which including octamer binding transcription factor 3/4 (OCT3/4), SRY-related high mobility group (HMG) box protein 2 (SOX2), MYC and Kruppel like factor 4 (KLF4) and by culturing these cells under ES cell conditions, the iPSCs can be generated from fibroblasts. The method is striking in that it can convert somatic cells directly into pluripotent cells by straightforward reprogramming procedure.
With the synthesis of scientific principles and technologies advancing, the induced pluripotent stem cells (iPSCs) have been developed over the last six decades. iPSCs are human somatic cells which have been reprogrammed to a pluripotent state, these cells can be considered as a potential patient-specific cell therapy or a specific model for disease research. With a sophisticated design, in Creative Biolabs, the iPSCs can be used as the pluripotent starting material for differentiated cells or tissues in regenerative medicine and they can be used as a powerful tool for immune cells treatment.
With efforts made by our brilliant scientists, at Creative Biolabs, we provided iPSCs services to clients all over the world.
iPSC derived stem cell therapy

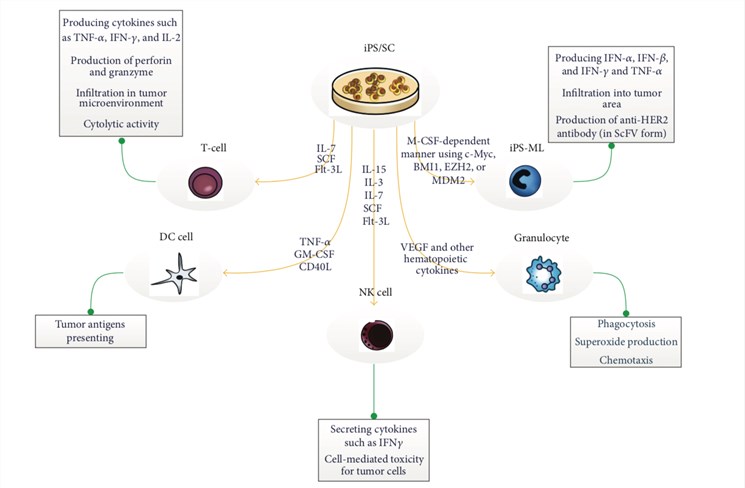
iPSC derived immune cell therapy
Currently, various platforms have been established to generate and analyze iPSC-derived immune cells to promote the development of novel stem cell therapy. We can provide the following custom services based on different immune cells:
Please contact us without hesitation to get more information.
References
For Research Use Only. Not For Clinical Use.