GTOnco™ MHC-associated Peptide Proteomics Assay
Immunogenicity is a key factor influencing the safety and efficacy of gene-therapy-based I-O products, such as peptide vaccines or T-cell vaccines for immunotherapy. MHC associated peptide proteomics (MAPPs) assay uses liquid chromatography / mass spectrometry (LC/MS) to identify peptide sequences which are presented by MHC on antigen-presenting cells (APCs) and therefore may induce the immunogenicity. MAPPs assay can be also used for determining epitopes that can be deimmunized. As a leading service provider in the field of I-O drugs development, Creative Biolabs provides top-quality MAPPs assay services for our customers to directly identify the peptides presented by APCs to T cells.
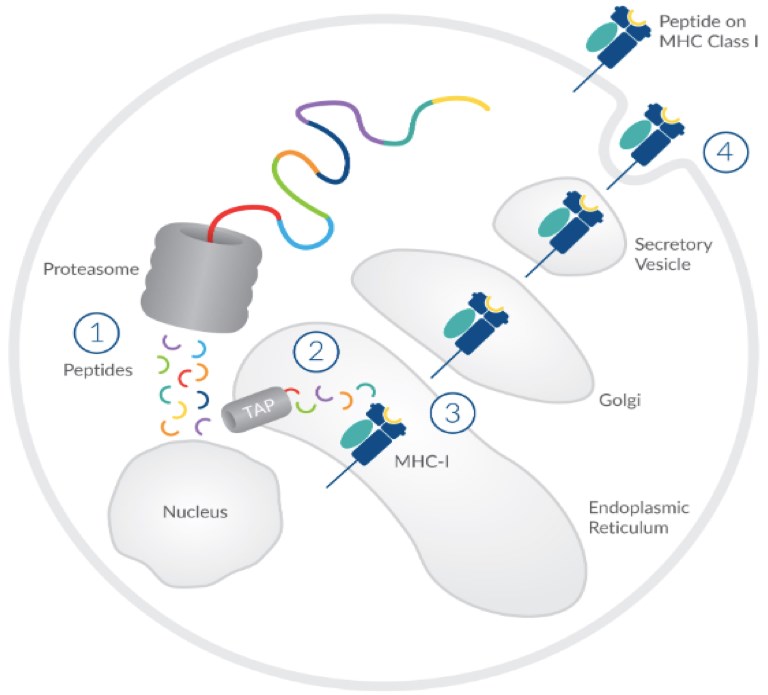 Figure 1. Stages of MHC-I Peptide Presentation. (Pisano, 2017)
Figure 1. Stages of MHC-I Peptide Presentation. (Pisano, 2017)
In the adaptive immune cycle, antigens or therapeutic proteins are first internalized by APCs and then degraded into short peptides. At the same time, these short peptides are presented by MHC molecules of the APCs. The resulting MHC-peptide complexes are further recognized by specific T cells and the activation of these cells triggers the immune responses. The sequence of MHC-associated peptide has a large impact on the initial process of this immunogenic pathway.
At Creative Biolabs, we have successfully established an MHC associated peptide proteomics system to predict and assess T cell epitopes in amino acid sequences. In GTOnco™ MAPPs assay, the therapeutic I-O agent is co-incubated with APCs and the peptide fragments are further presented on MHC molecules. Then, the cells will be lysed and MHC-associated peptides are then extracted and purified. Finally, the amino acid sequences of the peptide will be analyzed by LC-MS/MS. A series of stringent criteria are applied to implement quality control of our assay process in order to guarantee reliability and identify true positive peptides.
Creative Biolabs manifests as a world-leading expert in I-O drugs discovery field and provides our clients with best and timely services to complete each project successfully. We are happy to share our advanced platforms, technologies, and resources to facilitate your projects and boost the progress to clinical trials. For more detail information, please feel free to contact us.
References
- Sekiguchi, N.; et al. (2018). MHC-associated peptide proteomics enabling highly sensitive detection of immunogenic sequences for the development of therapeutic antibodies with low immunogenicity. mAbs. 10(8), pp.1168-1181.
- Pisano, M. and Ford, M. (2017). Mass Spectrometric Characterization of Peptides Associated with Molecules of the Major Histocompatibility Complex. BioTechniques. 63(2).
