GTOnco™ Redirected Cytotoxic Activity Assay
Gene therapy-based I-O products including the bispecific antibodies and chimeric antigen receptor T cells (CAR-T) have been used for the treatment of various malignancies. These approaches take advantage of T-cell potency in cancer therapy by redirecting them against tumors. Importantly, unlike traditional therapies, CAR-T and bispecific antibody approaches circumvent the TCR/HLA-peptide restriction by creating artificial T cell recognition of more general tumor-associated antigens (TAA). Based on advanced technology and years of research, Creative Biolabs has the ability to conduct high-quality redirected cytotoxic activity assay to test the lymphocyte anti-tumor activity. We offer one-stop services for our clients in the redirection of T cells with bispecific antibodies (CD3, CD19) or CAR.
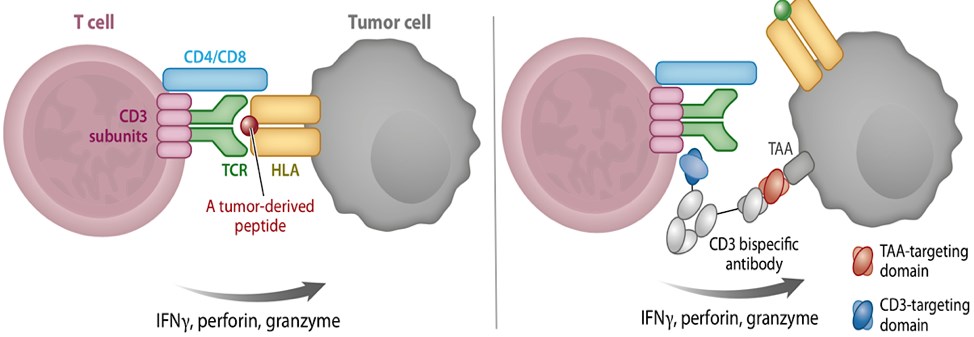 Figure 1. Redirection of T cells with CD3 bispecific antibodies. (Clynes, 2019)
Figure 1. Redirection of T cells with CD3 bispecific antibodies. (Clynes, 2019)
In the process of natural T cell recognition of tumor cell, the TCRs identify the tumor-derived peptides presented by HLA on the tumor cell surface, promoting T cell activation and the release of cytotoxic mediators. Generally, the approaches are based on the redirection of T cells, for instance, the CD3 bispecific antibody circumvents HLA restriction by simultaneously cross-linking a CD3 component of the T cell receptor complex with a TAA on the surface of the target tumor cell. These methods achieve T cell activation by mimicking the cognate TCR/HLA-peptide interaction.
At GTOnco™, we have developed systematic approaches to assess the redirected cytotoxic activity. In our assay process, the human unstimulated T cells isolated from PBMCs will be co-cultured with the I-O drug treated tumor cells to determine the redirection of T cells and assess the tumor lytic activity of I-O drug in the presence of human T cells. We provide a panel of positive cancer cell lines to confirm the anti-tumor activity and the ration of co-cultured T cells and targeted tumor cells will be also optimized in the assay.
As a frontier biotech service provider, Creative Biolabs is dedicated to providing the best-characterized in vitro assay service for our clients' gene therapy-based I-O products development. All tests are conducted by experienced technicians with the most advanced techniques. From the foremost project design to the final data interpretation, we implement strict inspections and validations on each and every step. Please feel free to contact us for further discussion with our scientists. Looking forward to cooperating with you.
Reference
- Clynes, R. and Desjarlais, J. (2019). Redirected T Cell Cytotoxicity in Cancer Therapy. Annual Review of Medicine. 70(1), pp.437-450.
