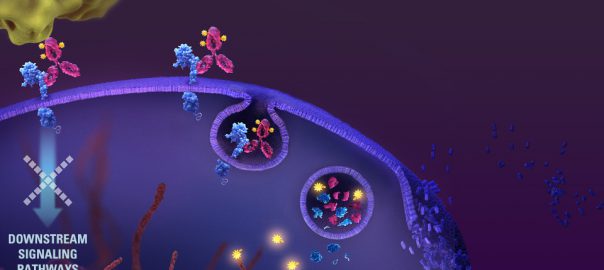
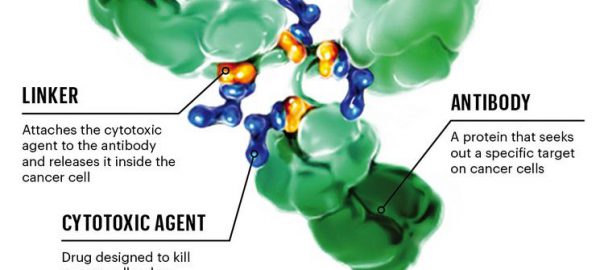
The United States FDA approved the extended indication of its antibody coupling drug Kadcyla (ado-trastuzumab emtansine) as a postoperative adjunctive therapy for early mammary cancer patients with positive HER2 and still residualRead More…
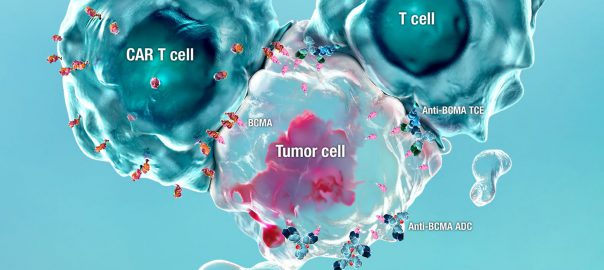
BCMA targeted therapy! GSK antibody drug conjugates apply for sale at the end of the year
British pharmaceutical company GlaxoSmithKline (GSK) recently announced the clinical study DREAMM -1 (NCT02064387) further positive data of experimental anti-B cell mature antigen (BCMA) antibody drug conjugate (ADC) GSK2857916 in the treatment ofRead More…
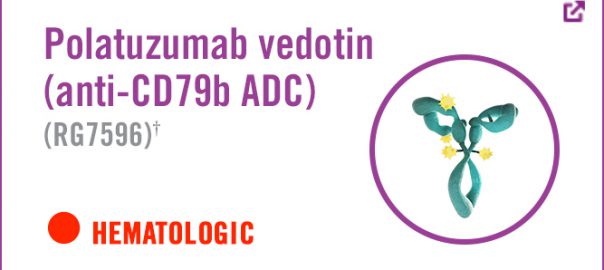
ADC-Polatuzumab Vedotin targeting CD79b has been prioritized by FDA in the United States
Roche recently announced that the Food and Drug Administration (FDA) has accepted the biological product licensing application (BLA) of the antibody drug conjugate (ADC) Polatuzumab Vedotin and granted priority review qualifications forRead More…
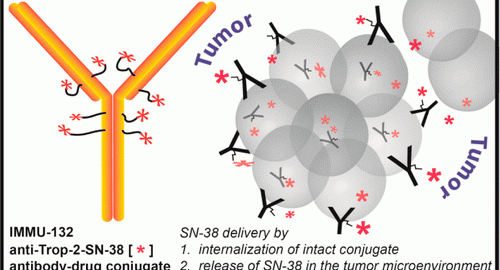
Immunomedics triple negative breast cancer ADC drug rejected by FDA in the United States
Immunomedics is a clinical biopharmaceutical company specializing in the development of monoclonal antibody-based therapies for the targeted treatment of cancer and other serious diseases. Recently, the company’s antibody-drug conjugate (ADC) sacituzumab govitecanRead More…
ADC drug Based on Photoimmunotherapy Secured $284 Million in Series C Financing
Recently, Rakuten Aspyrian, a clinical stage biotechnology company, announced a total of $284 million in Series C funding round with its antibody-drug conjugates based on photoimmunotherapy, including $134 million jointly funded byRead More…
ADC Drug DS-8201 Shows Strong Efficacy in Patients with Low HER2 Breast Cancer Expression
Breast cancer is the most common cancer and cause of death among women. About 20% of breast cancers are HER2-positive (IHC3+ or IHC2+/ISH+), and the remaining 80% are HER2-negative. HER2 is aRead More…
FDA Grants Orphan Drug Designation to STRO-001 (ADC) by Sutro
Recently, Sutro Biopharma announced that the US Food and Drug Administration (FDA) has granted STRO-001 the orphan drug qualification for the treatment of multiple myeloma (MM). STRO-001 is a potential first antibodyRead More…
The Success of Seattle Genetics/Wutian ADC Adcetris for Phase III Clinical Surgery of Peripheral T Cell Lymphoma (PTCL)
October 03, 2018 – Seattle Genetics and Takeda recently announced the evaluation of antibody conjugates Adcetris (brentuximab vedotin) for the treatment of peripheral T-cell lymphoma (PTCL, also known as mature Phase IIIRead More…
Seattle Genetics Launched the Phase II Clinical Trial of Antibody-drug Conjugate (ADC) Called Enfortumab Vedotin
Seattle Genetics and its partner, Astellas, have recently announced that the patients were selected for EV-201, the key phase II clinical trial of an experimental antibody-drug conjugate (ADC) called enfortumab vedotin. TheRead More…
FDA Grants Priority Review for Sacituzumab Govitecan for the Treatment of Metastatic Triple-negative Breast Cancer
On July 18, Immunopharmas, a leading biopharmaceutical company in the field of antibody-drug conjugates, announced that the Biologics License Application (BLA) of its sacituzumab govitecan for the treatment of metastatic triple-negative breastRead More…