OUR SOLUTIONS
Innovative design is the cornerstone of successful bispecific antibody development. At Creative Biolabs, we combine computational expertise with extensive antibody engineering experience to provide comprehensive bispecific antibody design solutions that lay the foundation for successful therapeutic development.
Comprehensive Design Package
-
Detailed molecular design specifications
-
Structure visualization and analysis
-
Developability assessment report
-
Manufacturing feasibility evaluation
-
Design optimization recommendations
Advanced Design Technologies
-
AI-powered protein structure prediction
-
Molecular dynamics simulations
-
Advanced modeling of protein-protein interactions
-
Computational stability assessment
-
Design optimization algorithms
Target-Based BsAb Design
-
Epitope analysis and selection for optimal target engagement
-
Structure-based design to optimize binding domain orientation
-
Interface engineering for enhanced stability and reduced immunogenicity
-
In silico assessment of target accessibility and binding compatibility
-
Prediction of potential cross-reactivity and off-target effects
Application-Based BsAb Design
Immune cell recruitment strategies
Creative Biolabs provides comprehensive purification solutions for bispecific antibodies
generated through various approaches, including recombinant expression, chemical conjugation,
and hybrid-hybridoma technology. Regardless of the bsAb format, our optimized workflow
guarantees high purity and yield, meeting the specific requirements of each product. Contact
our specialists to discuss your specific project requirements and how we can help optimize your
bispecific antibody purification
process.
01
Project Consultation and Design
-
Collaborate with clients to understand the specific format and project requirements.
-
Design a tailored purification strategy based on antibody structure, binding properties, and production scale.
-
Establish timelines and quality control checkpoints for the purification process.
02
Sample Preparation
-
Receive BsAb samples from clients or coordinate production within Creative Biolabs’ facilities.
-
Perform initial quality checks (e.g., concentration, aggregation analysis) to ensure the sample is ready for purification.
03
Capture Step – Primary Purification
-
Use affinity chromatography (e.g., Protein A) to isolate the target bispecific antibody.
-
Remove major impurities, including host cell proteins, DNA, and process-related contaminants.
-
Collect eluate fractions for further processing.
04
Polishing Step – Secondary Purification
-
Apply advanced purification techniques such as IEX, SEC, or HIC.
-
Refine the product to remove aggregates, isoforms, and residual impurities.
-
Ensure homogeneity, stability, and batch-to-batch consistency.
05
Quality Control and Analysis
-
Conduct rigorous analytical testing, including SDS-PAGE, HPLC, mass spectrometry, and bioassays, to verify purity and functionality.
-
Provide clients with a detailed certificate of analysis (COA) for each purified batch.
-
Formulation and buffer exchange (optional).
06
Packaging and Delivery
-
Package purified antibodies according to regulatory and client-specific guidelines.
-
Coordinate secure and temperature-controlled shipping to ensure product integrity upon delivery.
At Creative Biolabs, we leverage our proprietary antibody engineering platform and decades of
expertise to develop novel and functional bispecific antibodies. Our comprehensive engineering
capabilities enable the optimization of various molecular properties to meet specific
therapeutic requirements.
Bispecific IgGs represent a sophisticated class of engineered antibodies that maintain the canonical IgG structure while incorporating dual targeting capabilities. These molecules preserve the natural architecture of conventional antibodies, including the Fc region critical for effector functions and extended half-life, while enabling simultaneous binding to two distinct antigens.
-
CH3 domain engineering
-
Complementary interface mutations
-
Tyrosine knob/Threonine hole
-
Optional disulfide stabilization
-
Full IgG architecture
Engineered CH3 domains with sterically complementary interfaces (large knob into hole) to force heavy chain heterodimerization
-
High heterodimerization efficiency (>90%)
-
Robust production process
-
Stable heterodimer formation
-
Compatible with standard IgG production
-
Proven clinical success
-
Industrial scale feasibility
-
Dual targeting capability
-
Single molecule format
-
Multiple targeting strategies
-
Integrated direct/indirect actions
Combination of two targeting functionalities in a single IgG molecule through antibody engineering for direct or indirect therapeutic actions
-
Simplified development compared to combination therapy
-
Less complex manufacturing
-
Single molecule therapeutic
-
Multiple mechanism integration
-
Reduced clinical testing complexity
-
Full-length IgG structure
-
Engineered CH3 domain
-
Electrostatic steering mechanism
-
Natural antibody architecture
-
No artificial linkers
Assembly of two different antibodies through engineered complementary charged residues in CH3 domains and LC-HC interfaces
-
Exclusive heterodimer formation
-
Prevents LC-HC mispairing
-
Natural IgG properties
-
Commercial manufacturing compatibility
-
High stability
-
Good bioavailability
-
Maintained pharmacokinetics
-
IgG1-based structure
-
CH3 domain mutations
-
Wild-type IgG1 hinge
-
Controlled redox process
-
Natural antibody architecture
Controlled redox-driven exchange of half-antibodies through engineered CH3 domains enabling unidirectional recombination
-
High product homogeneity
-
Efficient exchange process
-
Post-exchange stability
-
Natural IgG properties retained
-
Physiological stability
-
Scale-up compatibility
-
Heterodimeric Fc platform
-
Human IgG/IgA CH3 hybrid
-
[IgG1 hinge]-CH2-[SEED CH3] structure
-
Asymmetric design capability
Uses asymmetric strand exchange between CH3 domains to drive heterodimerization
-
Preferential heterodimer formation
-
Retained Fc properties
-
Rapid protein A purification
-
Flexible fusion options
-
Half-life extension
-
Avoids light chain mixture complications
-
Multiple fusion partner capability
-
Fully natural structure
-
Common heavy chain
-
Distinct κ and λ light chains
-
Unmodified IgG architecture
Exploits natural selectivity of κ and λ light chains for their respective heavy chains
-
Identical to human antibodies in properties
-
High-level expression
-
Extended half-life
-
Natural effector functions
-
Low immunogenicity risk
-
Engineered heavy chain-light chain interface
-
Native antibody geometry
-
Orthogonal CH1-CL or VH-VL interfaces
-
Structure-based design
Creates orthogonal interfaces between heavy and light chains to ensure correct pairing
-
Eliminates light chain mispairing
-
Maintains native Fab stability
-
Minimal impact on antigen binding
-
Applicable across multiple germline segments
-
Natural antibody-like properties
Appended IgGs combine the conventional IgG structure with additional binding domains to enhance functionality. These molecules are engineered by strategically appending additional binding moieties, such as scFv or Fab fragments, to different positions of the base IgG structure.
-
IgG backbone with scFv fusion
-
Tetravalent molecule
-
Dual targeting capability
Fusion of single-chain variable fragments to the heavy or light chains while maintaining intact IgG structure
-
Homogeneous product formation
-
Easy protein A purification
-
Multiple targeting options
-
Maintained IgG functionality
-
Design flexibility
-
Stable structure through disulfide stabilization
-
Four possible formats (H/L, N/C terminal)
-
Full IgG scaffold
-
Additional Fv domains
-
Maintains mAb features
Direct fusion of individual variable domains to IgG structure
-
Simultaneous binding to all variable domains
-
Higher specific binding capacity
-
Extended epitope distance
-
Natural antibody avidity retention
-
Flexible format selection
-
Maintained Fc functions
-
IgG with tandem variable domains
-
Natural IgG flexibility
-
Short peptide linkages
-
Dual Fv regions in both H and L chains
Integration of variable fragments at N-terminus with independent heavy and light chain components
-
Maintains structural freedom for dual binding
-
Full IgG functionality (ADCC, CDC)
-
Extended serum half-life
-
Natural antibody-like properties
-
Optimizable through linker adjustment
-
Full-length IgG with C-terminal Fv
-
Asymmetric structure
-
Bivalent N-terminal + monovalent C-terminal binding
-
VH/VL domain appendages
Strategic placement of variable domains at C-terminus with separate heavy and light chain engineering
-
Different valencies for different targets
-
Enhanced heavy chain heterospecificity
-
Maintains natural IgG properties at N-terminus
-
Controlled binding geometry
-
Combined mono- and bi-valent targeting
-
scFab domain fused to C-termini of IgG
-
KIH-modified heavy chains
-
Asymmetric structure
-
2+1 or 2+2 binding valency options
Combination of knobs-into-holes technology with single-chain Fab fusion for enhanced stability
-
Prevents homodimer formation
-
Industrial scale production feasible
-
Higher specific binding capacity
-
Flexible binding valency
-
Extended epitope distance
-
Maintained natural antibody avidity
-
Four scFv binding sites
-
IgG-like heterotetramer
-
Dual specificity (2+2 format)
-
Natural dimerization mechanism
Integration of four single-chain variable fragments with immunoglobulin backbone
-
Homogeneous product formation
-
Simultaneous binding to two epitopes
-
Maintained component antibody activity
-
Enhanced binding avidity through bivalency
-
Enables receptor cross-linking/dimerization
-
Compensates for lower individual affinities
-
Natural antibody architecture
-
Tetravalent symmetric structure
-
Four Fab fragments + Fc domain
-
Optional peptide connectors
Strategic fusion of complete Fab fragments to IgG structure
-
Correct chain pairing and orientation
-
No unexpected by-products
-
Good expression yields
-
One-step purification
-
Comparable stability to natural antibodies
-
Maintained Fc functionality
-
Similar pharmacokinetics to parent antibodies
-
Appropriate flexibility for dual binding
Bispecific antibody fragments represent innovative molecular formats that eliminate the Fc region while retaining essential binding capabilities. Their reduced size compared to full-length antibodies facilitates enhanced tissue penetration and potential for novel delivery routes.
-
Derived from camelid heavy-chain antibodies
-
Small size (~15 kDa)
-
Humanized VHH sequence
-
Hydrophilic framework-2 modifications
Fusion of two single-domain antibodies through genetic engineering
-
Excellent tissue penetration
-
High solubility and stability
-
Monomeric behavior
-
Independent of Fc effects
-
Picomolar activity
-
No FcgRIIb engagement
-
Glycosylation independent
-
Easy to produce
-
Matches toxin peptide size
-
scFv pairs linked via CH3 domains
-
Intermediate molecular weight
-
Available in bispecific and Tribi formats
-
KIH engineered CH3
Fusion of scFv domains with CH3 domains for controlled dimerization
-
Optimal balance of tumor uptake and clearance
-
Enhanced avidity (especially Tribi format)
-
Improved pharmacokinetics over scFv
-
Stable heterodimer formation
-
Strong cytotoxicity at lower concentrations
-
Two scFvs connected in series
-
Four tandem variable regions
-
Optional CH3/Fc engineering
Expression in mammalian cells with variable regions connected by flexible peptides
-
Superior T cell recruiting efficiency
-
Ensures close target proximity
-
Flexible for engineering modifications
-
Can be extended to multi-specific formats
-
Modifiable half-life through Fc fusion
-
Smallest BsAb format
-
VH-VL fragments with short GGGGS linkers
-
Multiple engineered variants (DART, scDiabody, Diabody-CH3/Fc)
-
Rigid structure design
Forced dimerization through short linkers between variable domains
-
Predictable and rigid structure
-
Efficient dimerization
-
Small size
-
Multiple engineering possibilities
-
Can be modified for extended half-life
-
Three main constructs: scFv-CH3, scFv-Fc, and scFv-CH-CL-scFv
-
Single polypeptide design
-
(Gly4/Ser)3 peptide linker
-
Fundamental building blocks
Fusion of VH and VL domains through a flexible peptide linker
-
Overcomes chain-pairing problems
-
Minimal homodimer contamination
-
Flexible design options
-
Simple polypeptide structure
-
Efficient target recognition
-
Fab scaffold with additional functional entities
-
C-terminal extensions preferred
-
Multiple specificity options (1+1, 2+1, 1+1+1)
-
Various fusion possibilities
Combination of three binding domains in single molecule
-
Versatile fusion options
-
Natural chain pairing mechanism
-
Multiple targeting capabilities
-
Flexible functionality integration
-
Compatible with various expression systems
-
Natural Fab architecture
-
CH1-CL stabilizing domains
-
Novel Fab interface design
-
Chemical coupling capability
Expression of Fab moieties with engineered interfaces for proper HC/LC pairing and heterodimerization through Ellman's reaction
-
Higher thermal stability than scFv formats
-
Minimal aggregation tendency
-
Natural antigen-binding properties
-
Expression in both prokaryotic/eukaryotic systems
-
Efficient purification
-
Strong biophysical properties
-
Natural Fab pairing mechanism
-
Optional Fc engineering with KIH
-
Intermediate-sized molecules
Integration of scFv and Fab fragments utilizing natural pairing mechanisms and optional Fc engineering with KIH technology
-
Minimizes homodimer formation
-
Balanced molecular size
-
Better tissue penetration than full antibodies
-
Optional Fc functions
-
Natural pairing mechanism
-
Efficient renal clearance avoidance
-
Camelid-derived heavy chain only structure
-
Tandem VHH domains
-
Human IgG2/IgG3 constant regions
-
Lacks CH1 domain
Expression of artificial bispecific chains (VHH1-hCH2-hCH3-VHH2) with dimerization through human IgG constant regions
-
Outstanding tissue penetration
-
High physical/chemical stability
-
Maintained parental specificity
-
Enhanced efficacy at lower doses
-
Simple structure
-
Small size
-
Identical immunoglobulin chains with Fc domains
-
Two pairs of distinct scFvs
-
Tetravalent bispecific format
Dimerization through recombinant Fc domains with scFvs linked to each end
-
Simplified generation process
-
Tetravalent binding capacity
-
Uniform component structure
-
Bispecific and bivalent properties
Bispecific fusion proteins integrate antibody-derived fragments with various fusion partners including cytokines, growth factors, or albumin. This versatile platform enables the creation of multifunctional therapeutics.
-
Fusion of anti-CD3 scFv with affinity-matured TCR
-
HLA-peptide recognition capability
-
Immune synapse formation
-
T cell redirecting ability
Combination of high-affinity TCR recognition with anti-CD3 immune activation through engineered fusion protein design
-
Overcomes natural TCR affinity limitations
-
Enables targeting of intracellular antigens
-
Creates optimized immune synapse
-
Effective T cell recruitment
-
Enhanced tumor recognition
-
Fusion of BsAb with human serum albumin (67 kDa)
-
Multiple format options (tandem scFv-HSA, scDb-HSA, dual scFv-HSA)
-
Mammalian expression system
-
Natural serum protein fusion
Integration of small BsAb formats with HSA through genetic fusion to create long-circulating therapeutic proteins
-
Significantly extended serum half-life
-
Reduced dosing frequency
-
Maintained binding specificity
-
Enhanced pharmacokinetics
-
Natural carrier protein benefits
-
Strong stability
-
Fusion of BsAb with catalytic toxins (PE/DT)
-
Multiple targeting capability
-
Deimmunized toxin options
-
Specific receptor targeting
Combination of truncated toxins with dual targeting ligands through genetic engineering to create selective cancer-killing agents
-
Enhanced targeting through dual receptor binding
-
Selective cancer cell killing
-
Reduced damage to healthy cells
-
Superior to monospecific counterparts
-
Effective cytosolic delivery
-
Minimized immunogenicity
Bispecific antibody conjugates represent a sophisticated class of therapeutic molecules that combine antibody targeting with additional functional elements through chemical conjugation strategies, offering unique advantages in terms of modularity and functional diversity.
-
Multiple formats (IgG-IgG, F(ab')2, F(ab')3)
-
Chemical crosslinking approach
-
Hetero/homo-bifunctional reagents
Chemical conjugation of whole antibodies or fragments using specific crosslinkers (SPDP, Traut's reagent, o-PDM) through amino or sulfhydryl reactive chemistry
-
Flexible format selection
-
Bivalent binding capability
-
Controlled conjugation process
-
Format-dependent size options
-
Both reducible and non-reducible linkages possible
-
Chemical programming approach
-
Site-specific linkage
-
Multiple reactivity centers (U,C,K)
-
Scaffold antibody + peptide pharmacophore design
Integration of targeting peptides with antibody scaffolds through site-specific chemical conjugation using engineered reactive centers
-
Combined advantages of mAbs and peptides
-
Extended half-life
-
Maintained Fc functions
-
Unlimited cellular access
-
Enhanced receptor interference
-
Easier manufacture
-
High specificity
-
Multiple functional additions (PEG, fluorophores, siRNA, drugs)
-
Retained antibody reactivity
-
Additional reactive groups
-
Site-specific conjugation
Integration of antibodies with functional molecules through chemical crosslinkers containing multiple reactive groups for targeted modification
-
Extended half-life through PEGylation
-
Reduced inactivation risk
-
Better tissue penetrance
-
Maintained antibody function
-
Multiple functionality options
-
Site-specific modification control
We have established a comprehensive bispecific antibody analysis platform portfolio that integrates state-of-the-art technologies with extensive expertise in antibody characterization. Our sophisticated analytical strategies not only verify the unique dual-targeting capabilities of bispecific antibodies but also ensure their molecular integrity and therapeutic efficacy throughout the development process.
|
Analysis
|
Method/Technique
|
Description
|
|
Purity Measurement
|
Sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
|
SDS-PAGE Analysis separates proteins based on molecular weight using an electric field, where proteins bound with SDS migrate through a gel matrix, providing a straightforward method to assess antibody purity
|
|
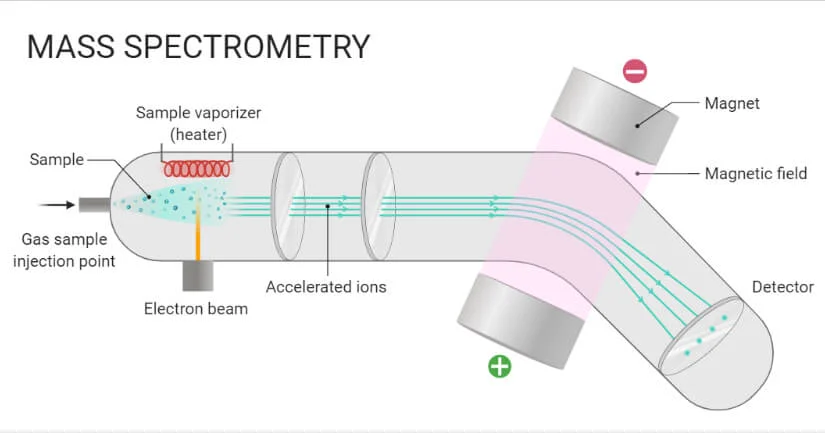
Liquid Chromatography-Mass Spectrometry (LC-MS)
|
LC-MS Analysis combines liquid chromatography for separation with mass spectrometry for detection, offering precise identification and quantification of antibody components and their structural properties.
|
|
Partial-filling Affinity Capillary Electrophoresis (PFACE)
|
PFACE analyzes binding interactions by measuring migration time changes in a capillary column, proving particularly effective for bispecific antibody purity assessment.
|
|
Molecular Weight/Mass Measurement
|
Size-exclusion Chromatography with Multi-angle Light Scattering (SEC-MALS)
|
SEC-MALS combines size-exclusion chromatography with multi-angle light scattering to determine absolute molecular weight and size of antibodies in solution. This technique measures both the absolute molecular weight and root-mean-square radius simultaneously, offering accurate measurements under native conditions.
|
|
Electrospray Ionization Mass Spectrometry (ESI-MS)
|
ESI-MS uses a "soft" ionization technique that can analyze large biological molecules like proteins without fragmenting them. This allows for measurement in their native forms and is particularly useful for biomolecules that can't be analyzed by conventional methods.
|
|
Electrospray Ionization Time-of-flight (ESI-TOF)
|
ESI-TOF ionizes molecules by creating charged droplets through an electric field, then measures their mass based on the time taken to reach a detector. This approach determines molecular mass by analyzing the mass-to-charge ratio of ions.
|
|
Molecular Structure Analysis
|
NMR Spectroscopy
|
NMR Spectroscopy utilizes magnetic properties of atomic nuclei to determine molecular structure and properties. This technique can analyze sample purity, identify compound structures, and study molecular conformations in solution, making it invaluable for detailed structural analysis of antibodies.
|
|
Small-angle X-ray Scattering (SAXS)
|
SAXS analyzes particles ranging from small peptides to large macromolecules. It provides low-resolution structural information and can quickly determine if proteins are properly folded or aggregated in solution, making it essential for rapid macromolecular characterization.
|
|
Molecular Docking
|
Molecular Docking predicts how molecules bind together and their binding affinity. Using various computational chemistry software like AutoDock and RosettaDock, it helps understand how antibodies interact with their targets and form stable complexes.
|
|
Analysis
|
Method/Technique
|
Description
|
|
Aggregation Analysis
|
Dynamic Light Scattering (DLS)
|
DLS measures protein aggregates by analyzing scattered laser light patterns. This technique determines particle size distribution in therapeutic formulations by detecting light fluctuations at specific angles, making it valuable for monitoring protein aggregation.
|
|
Electrospray Differential Mobility Analysis (ES-DMA)
|
ES-DMA measures soluble protein aggregates ranging from 3 to 250 nm. It excels at characterizing early aggregation states by electrospraying protein solutions and separating different aerosolized species, providing detailed size distributions of protein assemblies.
|
|
Sedimentation Velocity Analytical Ultracentrifugation (SV-AUC)
|
SV-AUC determines protein size distributions with minimal disruption to sample integrity. It analyzes proteins directly in their formulation buffer, making it particularly suitable for studying native protein states.
|
|
Field Flow Fractionation (FFF)
|
FFF separates and quantifies protein aggregates across a broad size range. This matrix-free technique effectively separates monomers from oligomers and serves as a complementary method to AUC analysis.
|
|
Thermal Stability Analysis
|
Differential Scanning Calorimetry (DSC)
|
DSC measures how physical properties of antibodies change with temperature variations. By detecting heat absorbed or released by the sample compared to a reference material, DSC can determine thermal transition temperatures in various phases, making it valuable for understanding biomolecule thermodynamic properties.
|
|
Differential Scanning Fluorimetry (DSF)
|
DSF uses fluorescent dyes to measure protein thermal stability. As proteins unfold with increasing temperature, the dye binds to exposed hydrophobic regions, allowing measurement of unfolding temperatures and assessment of protein-ligand binding stability. This technique is particularly useful for optimizing protein stability conditions.
|
|
Serum Stability Analysis
|
Enzyme-linked Immunosorbent Assay (ELISA)
|
ELISA measures serum stability by quantifying functional antibodies through antigen binding and color-change reactions. This technique uses immobilized antigens and enzyme-linked secondary antibodies to detect and measure the amount of intact, functional antibodies remaining in serum samples.
|
|
Flow-cytometry Assay
|
Flow cytometry assesses antibody stability by analyzing fluorescence-labeled antibodies binding to cells. This method enables simultaneous measurement of multiple parameters and can determine if antibodies maintain their ability to bind to specific cellular targets after serum exposure.
|
|
Analysis
|
Method/Technique
|
Description
|
|
Target Binding Affinity Measurement
|
Surface Plasmon Resonance (SPR)
|
SPR measures binding kinetics and affinities in real-time without labeling. It detects changes in resonant oscillations when molecules bind to a sensor chip surface, providing detailed information about antibody-target interactions.
|
|
Enzyme-linked Immunosorbent Assay (ELISA)
|
ELISA-based Assay uses color-change reactions to measure binding affinity. By detecting the concentration of bound antibodies to target antigens of known concentration, this widely-used technique can verify antibody-antigen binding strength.
|
|
Flow-cytometry Assay
|
Flow cytometry analyzes antibody-target interactions in a cell-based format. This method allows measurement of binding affinity in a natural cellular environment and doesn't require antibody purification, making it particularly useful for analyzing cell-surface targets.
|
|
ADCC Assay
|
51Cr Approach
|
The 51Cr approach measures ADCC by pre-loading target cells with radioactive chromium (51Cr). Cell lysis is quantified by measuring released radioactivity in the supernatant, providing a direct measure of antibody-mediated cell killing.
|
|
lactate dehydrogenase (LDH) Approach
|
The LDH approach measures ADCC by detecting LDH released from lysed cells. This simpler, non-radioactive method quantifies cell death by measuring LDH enzyme activity through colorimetric reactions.
|
|
CDC Assay
|
Classical CDC Assay
|
The classical CDC assay combines target cells with test antibodies and human serum (containing complement components). Cell death is measured using radioactive compounds that are released when cells are destroyed, indicating antibody-mediated complement activation.
|
|
Non-radioactive CDC Assays
|
Non-radioactive CDC assays measure cell death by detecting released cellular components like GAPDH using fluorescent or luminescent methods. These provide a safer alternative to radioactive assays while maintaining effective measurement of antibody-mediated complement killing.
|
|
ADCP Assay
|
Traditional ADCP Assays
|
Traditional ADCP assays require macrophages differentiated from primary monocytes over a week-long culture period. These assays use flow cytometry to measure phagocytic activity by distinguishing between labeled target cells and macrophages.
|
|
The Reporter-gene Bioassay
|
The reporter-gene bioassay is an innovative approach using engineered cells expressing FcγRⅡa and NFAT-RE/luc2. This provides a faster, more reliable measurement of ADCP activity by mimicking the natural signaling pathway of macrophage-mediated phagocytosis.
|
|
Analysis
|
Description
|
|
Antibody Absorption and Distribution
|
We analyze antibody absorption rates via multiple administration routes (IV, SC, IM) and determine bioavailability. Our services include plasma concentration measurement and tissue distribution analysis using advanced imaging and bioanalytical methods.
|
|
Antibody Metabolism and Elimination
|
We evaluate antibody degradation pathways and clearance mechanisms through half-life (t1/2) determination and proteolytic analysis. Our team measures clearance rates and examines elimination routes, including analysis of target-mediated drug disposition (TMDD).
|
|
Study Type
|
Description
|
|
In Vitro Studies
|
Our in vitro testing services encompass multiple model systems for comprehensive evaluation of bispecific antibodies. We perform metabolic analysis through cell-based assays to determine potency and drug-like properties, conduct transporter studies using specialized cell lines (including immortalized and transfected cells), hepatocytes, and membrane vesicles, and provide high-throughput ADME screening that covers liver microsomal stability, permeability assessment, and CYP inhibition analysis.
|
|
In Situ and Ex Vivo Studies
|
We utilize sophisticated organ perfusion models to simulate in vivo conditions, with a particular focus on liver perfusion studies for detailed analysis of absorption, transport, and metabolism. Our ex vivo analysis capabilities include tissue examination for enzyme and transporter expression changes, combined with comprehensive assessment of protein binding and hepatocyte stability to provide crucial insights into drug behavior.
|
|
In Vivo Studies
|
Our in vivo testing platform delivers thorough evaluation of bispecific antibodies through multiple animal models and analytical approaches. We conduct comprehensive PK/PD studies primarily in rat models, utilize engineered mouse models for specific CYP enzyme analysis, and perform detailed investigations of drug distribution and elimination pathways, complemented by drug-drug interaction evaluation and sophisticated DMPK modeling.
|
Leveraging our extensive experience and advanced technology, we offer three powerful bispecific antibody manufacturing strategies: genetic engineering, hybrid-hybridoma technology, and chemical conjugation. Our expert team is committed to delivering high-quality, scalable BsAb production services to support your therapeutic development needs.