Preventing cancer metastasis remains one of the main goals of current research, as most malignant cells can metastasize by exploiting abnormal leakage from blood vessels. In a recent study published in the international journal APL Bioengineering entitled “Interacting with tumor cells weakens the intrinsic clockwise chirality of endothelial cells”, scientists from the University of Michigan-Dearborn and other institutions have revealed the molecular mechanisms underlying the arrangement of vascular endothelial cells in the spread of cancer and the key role they play.
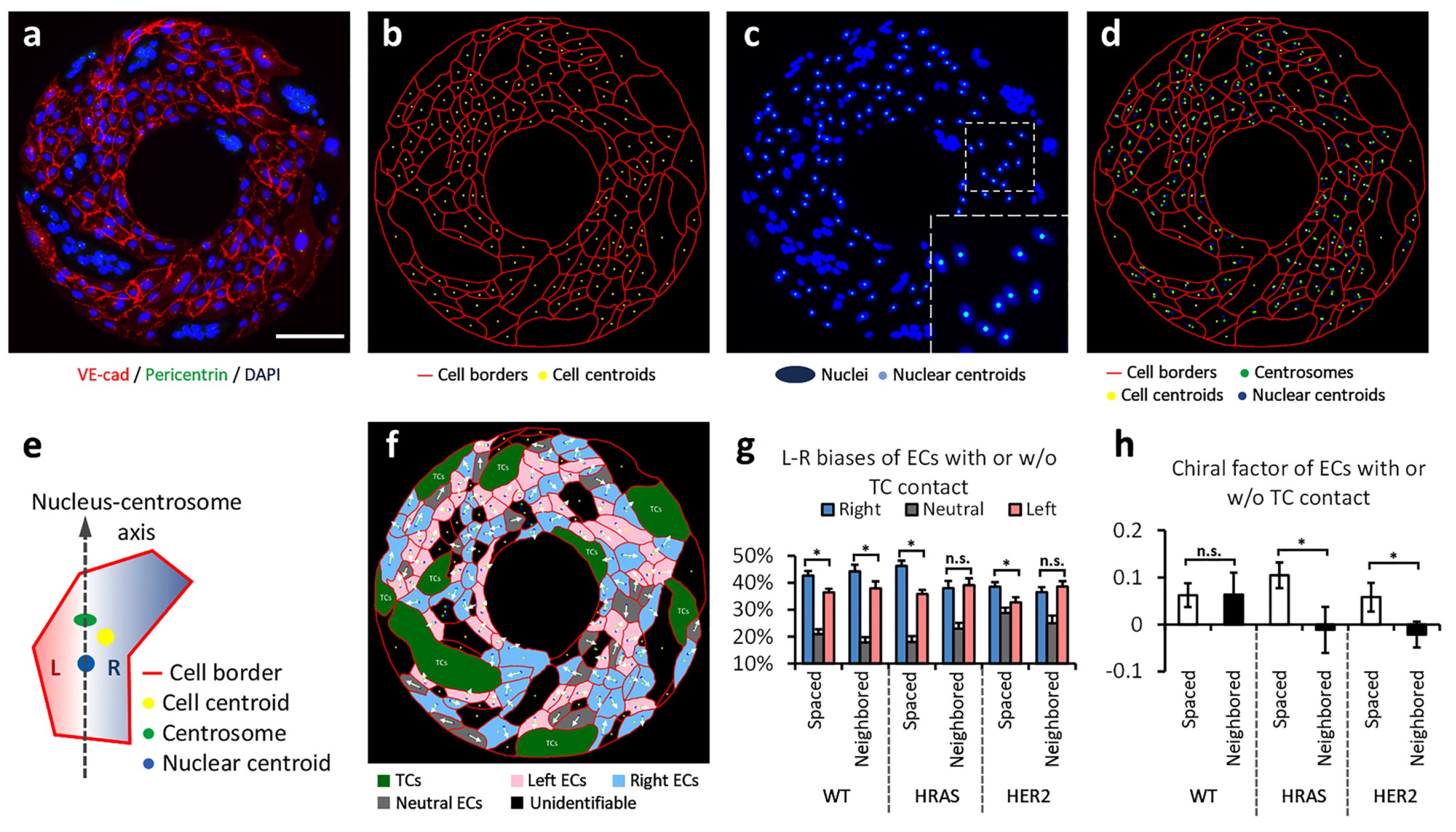
Fig.1 Interacting with tumor cells weakens the intrinsic clockwise chirality of endothelial cells. (Hang, 2022)
In this study, the researchers developed a novel model that may help analyze local communication between endothelial and tumor cells and its effect on endothelial cell alignment, primarily by using co-cultured human umbilical vein endothelial cells (HUVECs) and human mammary epithelial tumor cell lines to stimulate tumor-endothelial cell interactions. The researcher Jie Fan introduced that vessels in tumor tissue are more prone to leakage than those in normal tissue, and they were curious to see if tumor cells could induce a clockwise weakening effect on endothelial cells and induce a disruption of their function in the vasculature.
This study extends the recent findings that endothelial cells may have some chirality and tend to be more clockwise oriented; chirality is a mirror-image orientation characteristic similar to left and right handednes. The researchers suggest that the strong clockwise chiral characteristic of endothelial cells is important for maintaining vascular integrity, but unfortunately, it can be damaged or diminished by tumor cells, thus increasing the risk of cancer cell metastasis; while preserving this normal chiral characteristic may inhibit tumor metastasis by enhancing the integrity and chirality of the endothelial cell barrier in blood vessels.
To address this issue, the researchers used a series of tumor cell lines of varying malignancy to co-culture with HUVECs, and they subsequently compared how HUVECs responded when the tumor cells were in direct contact with them or not, and to exert this degree of control, the researchers employed a contact printing technique to create donut or figure-of-eight shaped micropatterns to contain the cells. The researchers found that the clockwise chirality of HUVECs was affected by local hormonal signals, and somewhat more so by direct physical contact with tumor cells. Specific proteins on tumor cells binded to proteins on endothelial cells, which appear to play an important role in altering the clockwise chirality characteristics of HUVECs.
The researchers were surprised by the movement of these cell types, which in most metastatic models run toward the blood vessels before the tumor cells break up and enter the bloodstream. The researchers hypothesized that the tumor cells would invade the endothelial cells of the body, yet they found that the endothelial cells were moving toward the tumor cells on the microscopic images. This interaction may help better control cancer metastasis, and they hope to further investigate the development of novel therapies.
In summary, the results of this study reveal that endothelial-tumor cell physical interactions play an important role in the chiral properties of endothelial cells, and that weakening endothelial cell chirality may potentially compromise the overall integrity of endothelial tissue, thereby increasing the likelihood of metastatic cancer spread.
Reference
1. Hang, Benson, et al. “Interacting with tumor cells weakens the intrinsic clockwise chirality of endothelial cells.” APL bioengineering 6.4 (2022).
