Fig. 1 BCL2 inhibition reveals a dendritic cell-specific immune checkpoint that controls tumor immunosurveillance (Zhao, 2023)
Recently, a team of researchers from the Gustave Roussy Cancer Research Institute in France published a groundbreaking study in the journal “Cancer Discovery.” This study reveals, for the first time, that BCL-2 serves as an immune checkpoint that restrains the function of human type I DCs (cDC1). Knocking out BCL-2 or using BCL-2 inhibitors can enhance the activation and antigen-presenting functions of cDC1. This approach synergizes with PD-1 inhibitors and holds promise for the treatment of solid tumors.
The researchers were able to identify BCL-2 by employing CRISPR/Cas9 technology. However, their screening target was not mature DCs in a terminal differentiated state but rather immortalized immature DCs that can proliferate indefinitely. These cells share overall transcriptomic characteristics with mature DCs and allowed researchers to accurately identify genes affecting crucial DC functions, cell cycle, and apoptosis.
BCL-2 emerged as a prominent candidate during the screening of genes related to antigen presentation. Additionally, BCL-2 is associated with DC apoptosis and is a readily available target, making it a natural focus of exploration.
Preliminary experiments have shown that using venetoclax or other highly selective BCL-2 inhibitors in development can enhance antigen presentation functions in immortalized immature DCs or bone marrow-derived DCs. However, the effectiveness of BCL-2 inhibitors relies on the autophagy of DCs and is ineffective in DCs with defective autophagy (Atg5/Atg7 gene knockout).
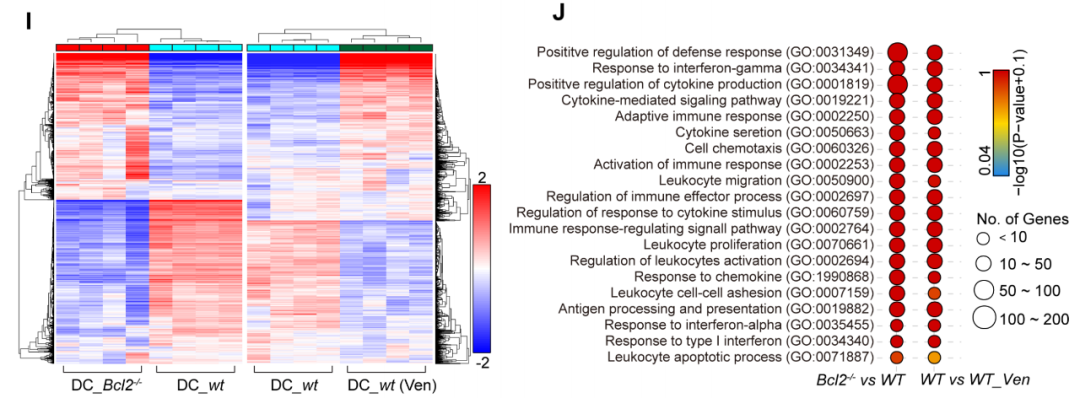
Knocking out BCL-2 or using BCL-2 inhibitors led to the upregulation of genes primarily (82%) associated with type I interferon responses in immortalized immature DCs. There was also a significant increase in the expression of chemokines (such as CCR2/CXCR3) and co-stimulatory molecules (such as CD80/83/86) receptors. Additionally, DCs exhibited enhanced secretion of interleukins like IL-1β and IL-6, all of which are favorable for enhancing the immunostimulatory properties of DCs.
Experiments in fibrosarcoma and non-small cell lung cancer (NSCLC) mouse models demonstrated that BCL-2 inhibitors could synergize with PD-1 inhibitors, more effectively inhibiting tumor growth. Analysis of changes in cell surface markers revealed that the DCs subgroup activated by BCL-2 inhibitors was primarily limited to cDC1 (evidenced by the upregulation of CCR7, XCR1, CD86, and MHC-II molecules).
Selective elimination of cDC1 or T cells, or the inhibition of DCs and myeloid cells from draining lymph nodes into tumor sites, abolished the immune-enhancing effect of BCL-2 inhibitors. This confirms the indispensable role of cDC1 in the effectiveness of BCL-2 inhibitors.
Furthermore, transferring a large number of BCL-2-knockout immortalized immature DCs into tumor-bearing mice enhanced cancer immune surveillance, dependent on T cells and type I interferon responses, effectively inhibiting tumor growth. Importantly, knocking out BCL-2 or using BCL-2 inhibitors did not significantly affect the survival of DCs, offering potential for a “transferred DCs therapy.”
Although the research team classifies the enhancement of DC function by BCL-2 inhibitors and the subsequent improvement of anti-tumor immune responses as “off-target effects,” these positive off-target effects are certainly welcome compared to various side effects. Previous studies have also shown that BCL-2 inhibitor treatment can improve the infiltration of effector T cells into colorectal cancer. Perhaps, under the stimulation of BCL-2 inhibitors, DCs and T cells can work in tandem. Several early clinical studies are now assessing the combination of BCL-2 inhibitors and PD-1/PD-L1 inhibitors, and it is hoped that these studies will yield positive results.
Reference
1. Zhao, Liwei, et al. “BCL2 Inhibition Reveals a Dendritic Cell–Specific Immune Checkpoint That Controls Tumor Immunosurveillance.” Cancer discovery 13.11 (2023): 2448-2469.
