CAR-T cell immunotherapy, the chimeric antigen receptor T-cell immunotherapy, is a highly efficient and advanced cell immunotherapy. The principle is to genetically modify the immune cell T cells in patients to kill cancer cells.
CAR-T cell immunotherapy has shown its striking effects in the treatment of leukemia, lymphoma, and multiple myeloma. At present, two CAR-T therapies have been approved by the FDA for marketing, namely Kymriah of Novartis for the treatment of patients under age 25 with relapsed or refractory acute lymphoblastic leukemia, and Yescarta of Kite Pharmaceuticals for the treatment of certain types of adult patients with large B-cell lymphoma.
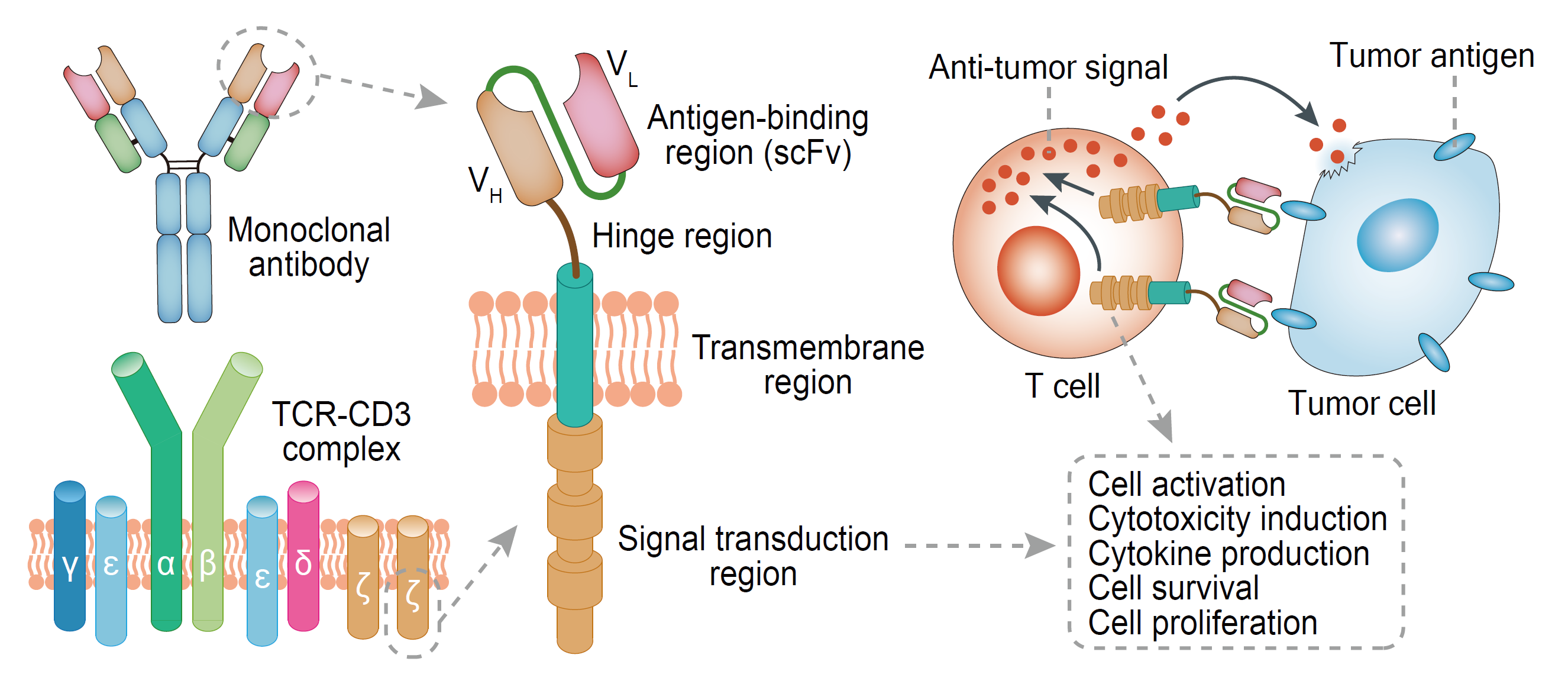
I CAR-T structure and anti-tumor mechanism
CARs consist of intracellular signaling regions (eg, CD3ζ and CD28), transmembrane regions, and extracellular antigen-binding regions of T cell receptors (TCRs), and the extracellular regions can identify specific tumor-associated antigen (TAA) with the function of single chain variable region fragment. Once CARs combine with TAA, T cells can exert effector function through the intracellular domain of CD3 or the high-affinity receptor FcεRI. Then T cells activate and proliferate as CTL cells, which can bind to tumor cells with TAA in the next encounter through the same mechanism and secrete perforin, granzymes and cytokines to synergistically kill the tumor cells. The CTL has a powerful enough ability to kill tens to hundreds of tumor cells.
II Evolution of the basic structure of CAR-T
In CAR-T therapy, single-chain antibodies that identify TAAs and fusion proteins of T cell activation sequences are expressed on the surface of T cells through the gene transduction technology. The first-generation CAR only carries the intracellular activation domain of CD3ζ and gradually evolves to the second generation CARs that carry intracellular co-stimulatory domains such as CD28/4-1BB/OX40/ICOS and intracellular activation domains of CD3ζ, and, to the third generation CAR that carries two co-stimulatory domains.
Faced with off-target effects resulting from the high heterogeneity of solid tumors and low specificity of tumor-associated antigen (TAA), some laboratory studies used a dual-signal CAR structure with two independent CAR structures, which separated the CD3ζ intracellular domain that provides the first signal of T cells activation, and the intracellular signal domain of the co-stimulatory receptor CD28/4-1BB/OX40 that provides the second signal. Then the two intracellular domains are connected to two independent CARs structures when two scFv antibodies targeting different TAA are used extracellularly, achieving a certain therapeutic effect against solid tumor animal models and effectively preventing off-target effects.
Based on this structure, some teams have also optimized extracellular single-chain antibodies, allowing the two single-chain antibodies to have different affinities for TAA, such as low affinity for CARs that provide the first signal, and high affinity for the co-stimulatory CAR, which can improve the off-target protection to some extent. In addition, some teams have attempted a design called iCAR, linking the intracellular signal domain of the PD-1 receptor, which provides an immunosuppressive signal, to the CAR carrying the ScFv against normal cell antigens, thus forming a dual-signal CAR structure with the second or the third generation CARs that target TAA. It can effectively prevent side effects on normal tissues in animal models.
In addition, CAR structures still in the preclinical stage have the genes that carry immune regulatory factors of independent constitutive or inducible expression on the basis of the second or third generation and in the gene expression frame of the CAR, which is referred to as the fourth generation of CAR. This structure can play a certain role in the promotion of killing in the face of its micro-environmental immunosuppression and solid tumor high heterogeneity when targeting solid tumors.
For some patients who are not suitable for autologous CAR-T therapy due to physical reasons and poor immune cell activity after repeated radiochemotherapy, the universal CAR-T (uCAR-T) called the fifth-generation CAR-T is undoubtedly the best choice.
Therefore, the CAR-T structure has gradually progressed in solving its problems. It has evolved to five generations of CAR structure. The clinical trial application mostly focuses on the second and third generation CAR structures. Other CAR structures need to further optimizations and improvements to meet the clinical efficacy and safety requirements in practical applications.
III Targets of CAR-T R&D
At present, the targets for clinical application include EGFR, HER2, CD22, CD19, CD138, ROR1, BCMA, CD123, Mesothelin and so on.
Since CAR-T uses its extracellular single-chain antibody for targeted binding to tumor cell surface-associated antigen (TAA) or tumor-specific antigen (TSA), then target killing tumor cells through the activation of T cells by its intracellular domain. Therefore, the selection of target antigen is crucial for the specific killing effect. However, the fact that TSA is difficult to use on the surface of tumor cells (the specific expression of CD19 is also a factor of CAR-T’s success in the treatment of B-lineage leukemia and lymphoma) leads to serious effects due to off-target effects of a series of CAR-T that target TAA, such as typical failures in clinical trials targeting HER2.
To effectively target TSA/TAA, it is necessary to select different scFvs based on different antigen expression conditions and different cancer species. For example, for high-specificity antigens such as NY-ESO/CD19, high-affinity scFvs can be used to provide a strong binding ability to activate the killing ability more rapidly. In normal tissues, for tumor-specific TAAs with minimal expression, it is appropriate to use medium-affinity scFv to achieve side effects such as reducing the off-target effect. In addition, for the stubborn solid tumors closely related to microenvironment, the special strategies can assist scFv to improve targeting ability and effective formation of immune synapses, which will help CAR-T to better exert its killing effect. Meanwhile, the current dual signal independent CAR-T structure or bispecific CAR-T structure needs to be improved and considered in this respect.