All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
CD19 (Cluster of Differentiation 19), also called B lymphocyte antigen CD19, is a protein that in human is encoded by CD19 gene. The human CD19 antigen is a 95 kd transmembrane glycoprotein. It is found on the surface of B cell, function as B cell co-receptor that assemble with B cell antigen receptor in order to decrease the threshold for antigen receptor dependent stimulation. CD19 is usually expressed on follicular dendritic cells and B cells. As a hallmark of B cell, CD19 has highly conserved expression on most B cell tumors, though there is no strong evidence to show if CD19 contributes to B cell carcinogenesis directly. It is expressed in most acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), and B cell lymphomas. In fact, the majority of B cell malignancies express CD19 at normal to high levels (80% of ALL, 88% of B cell lymphomas, and 100% of B cell leukemias). For these reasons, CD19 is also a potential target for lymphoma therapy. Several monoclonal antibodies and other antibody-based immune therapy such as CAR-T technique are targeted at CD19. It is foreseeable that CD19 mAb and molecularly engineered chimeric antigen receptor will be widely studied for therapies of lymphoma, leukemia, and autoimmune disorders.
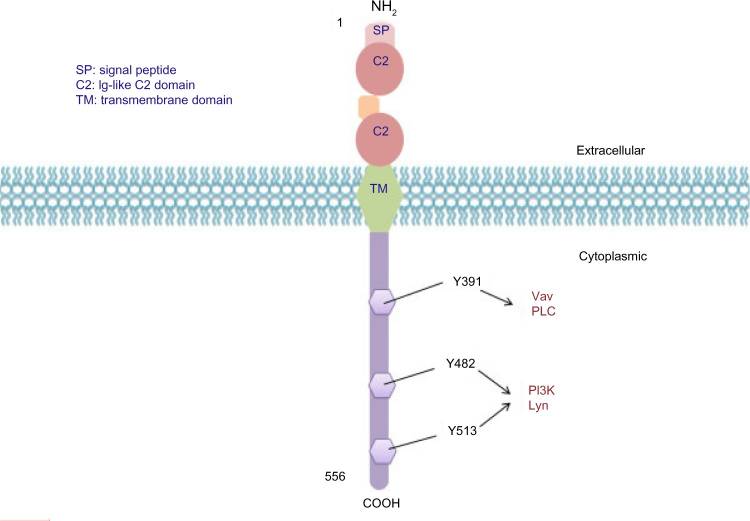
Fig.1 Structure of CD19 molecular.1
Creative Biolabs offers a complete series of CD19 protein products including biotinylated CD19, unconjugated CD19, and fluorescent-labeled CD19, which can efficiently identify the expression of anti-CD19 CAR on the surface of the transduced T cells and have been broadly employed for the quality control release testing and pharmacokinetic (PK) research of anti-CD19 CAR-T cells.
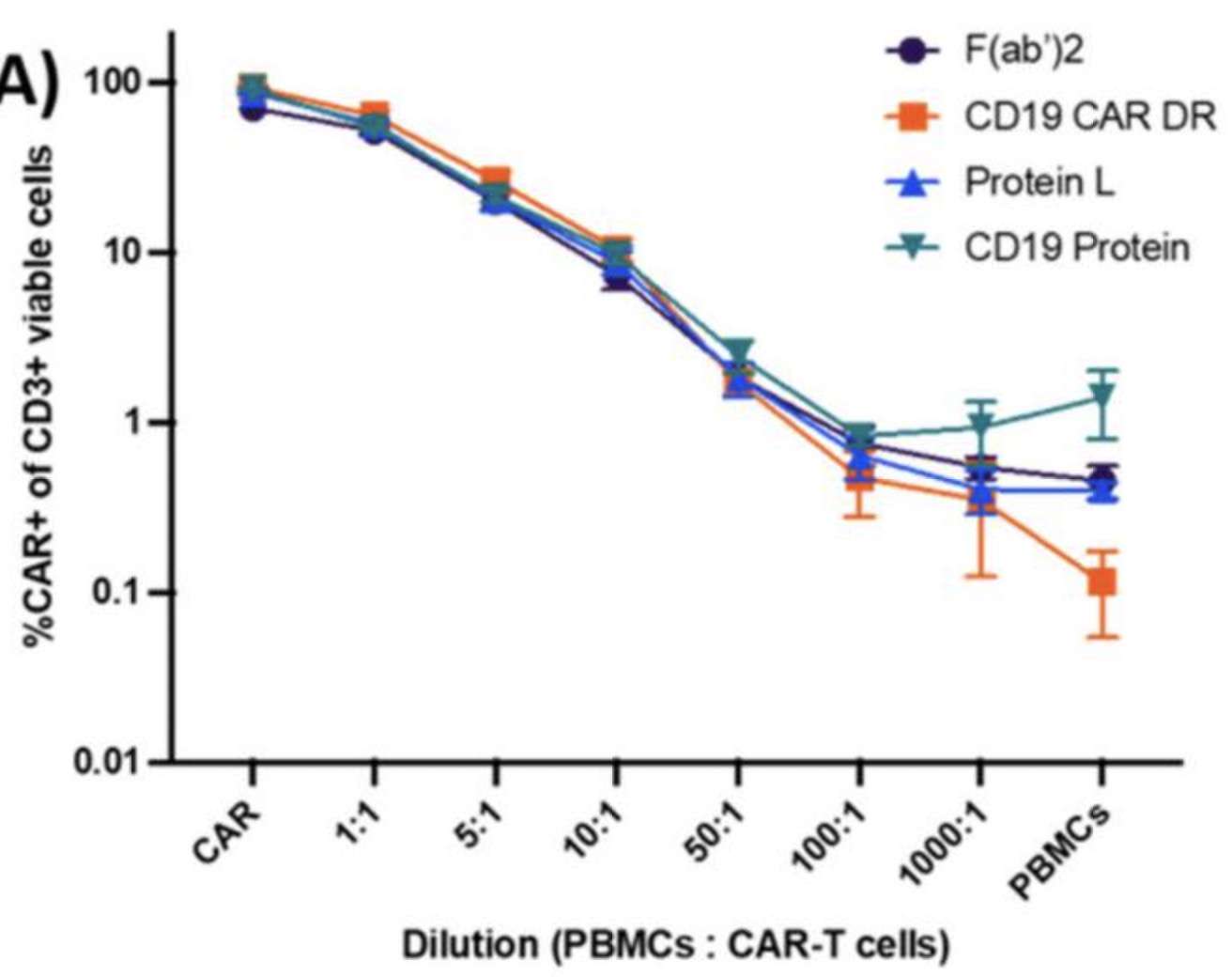
Fig.2 Sensitivity of the detection reagents to detect CD19-specific CAR-T cells in PBMCs (Schanda, et al., 2020). The figure shows CD19-specific CAR-T cells that were serially diluted in PBMCs of the same healthy donors at six different dilutions.
CD19 CAR-T Cytokine Release Test
The synchronous activation of CAR T cells encountering CD19-expressing leukemic cells and B lymphocytes directly results in cytokine release syndrome (CRS). Creative Biolabs provides unique cytokine release tests. For example, enzyme-linked immunosorbent assay (ELISA) to detect the secretion of Interferon gamma (IFNγ), Interleukin-2 (IL-2), and other cytokines.
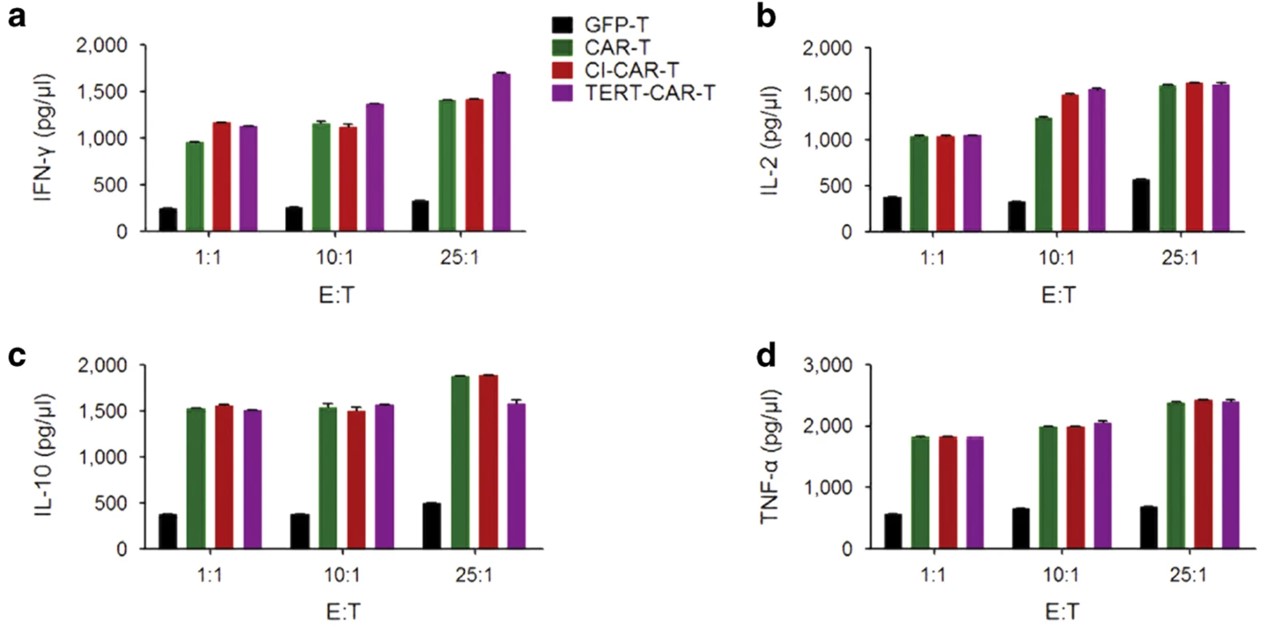
Fig.3 Cytokine release of TERT mRNA delivery to CD19 CAR-modified T cells.3 Four groups of T cells—GFP-T, CAR-T, CI-CAR-T, and TERT-CAR-T—were co-cultured with CD19+ Raji cells at varying E:T ratios. TERT mRNA-transduced CD19 CAR T cells exhibited cytokine secretion levels similar to those observed in the control groups
CD19 CAR-T In Vitro Cytotoxicity Assay
Anti-CD19 CAR-T lymphocytes therapy shows excellent effects in patients with B lymphocyte neoplasias, which can induce remission in young adults and children with lymphoid leukemia. Creative Biolabs offers proprietary cytotoxicity test for CD19 CAR-T cells. In CD19 CAR-T cells, the cytotoxic activity can be evaluated by luciferase assay and by lactate dehydrogenase (LDH) assay.
Targeting cell type of CD19 at Creative Biolabs includes:
T cell
NK cell
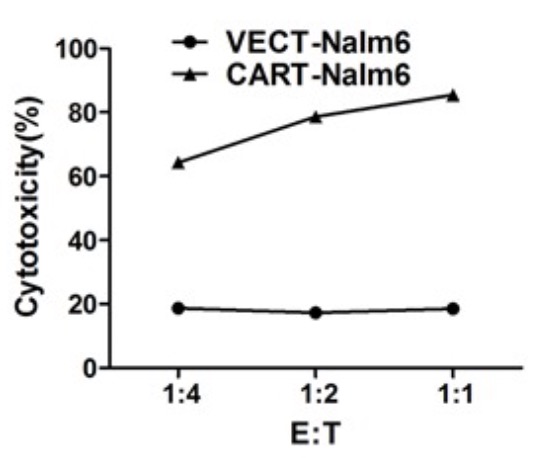
Fig.4 Cytotoxicity in CD19 CAR-T cells (An, et al., 2016). The cytolytic activity was measured by assays of lactate dehydrogenase (LDH) release after coculturing using the detection kit.
CD19 CAR-T Cell Proliferation Test
For evaluating our CAR products, Creative Biolabs has complete CAR testing services including cell proliferation tests to make sure the CAR-modified immune cells can be activated effectively once they encounter the target cells. The activation and proliferation process of CD19 CAR T cells can be measured via CFSE in vitro.
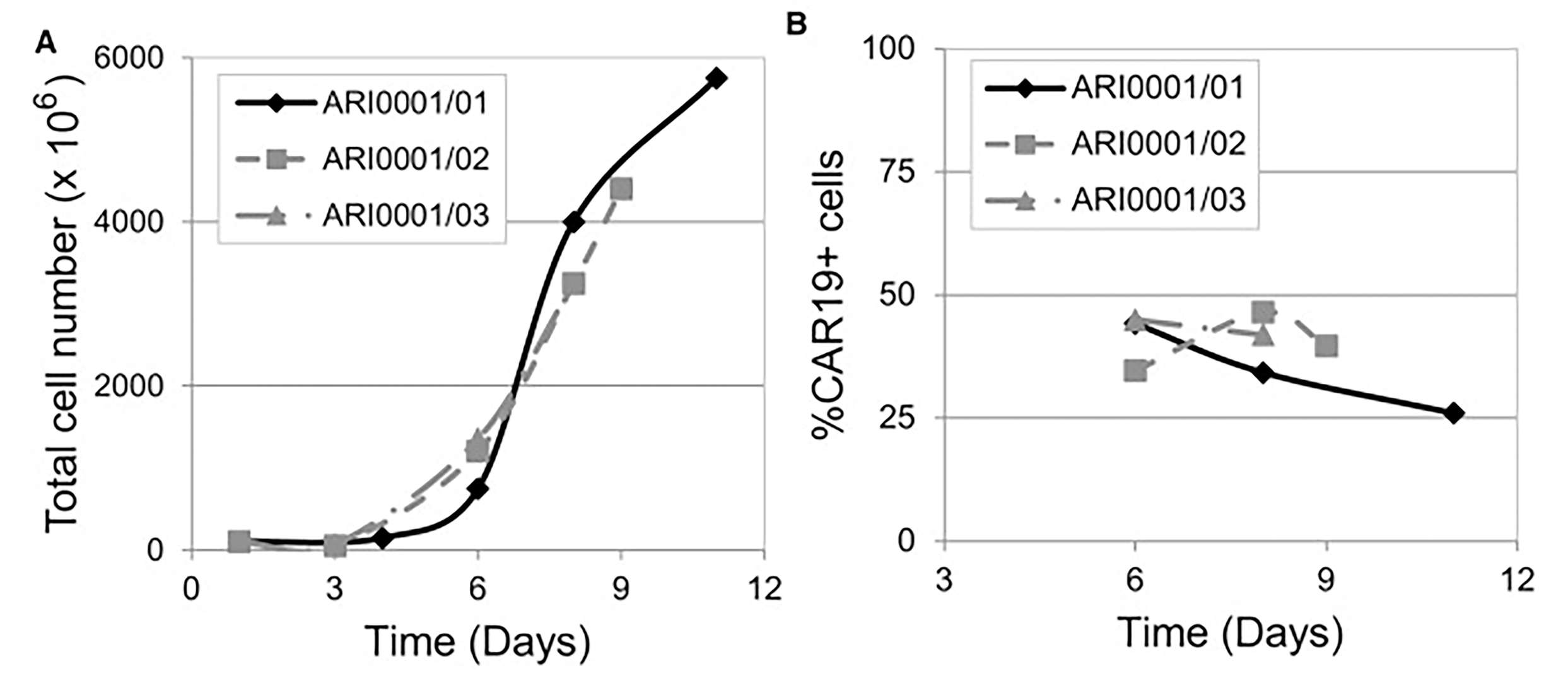
Fig.5 CD19 CAR-T cell proliferation (Castella, et al., 2018). A) Total cell number at different time points. B) Percentage of CAR19-expressing cells at different time points.
CD19 CAR-T Cell Therapy Animal Models
Creative Biolabs has established comprehensive efficacy models to facilitate in vivo efficacy evaluation including immune-oncology animal models, syngeneic models, humanized mice models, patient-derived xenografted (PDX) models, patient-derived organoid models, and cell line derived xenografted (CDX) models, such as raji xenograft model and MCL xenograft model for CD19 CAR-T therapy.
The human tumor cell lines of CDX models provided for CD19 CAR-T cell therapy at Creative Biolabs include:
NAML6 cell line
Raji cell line
MCL cell line
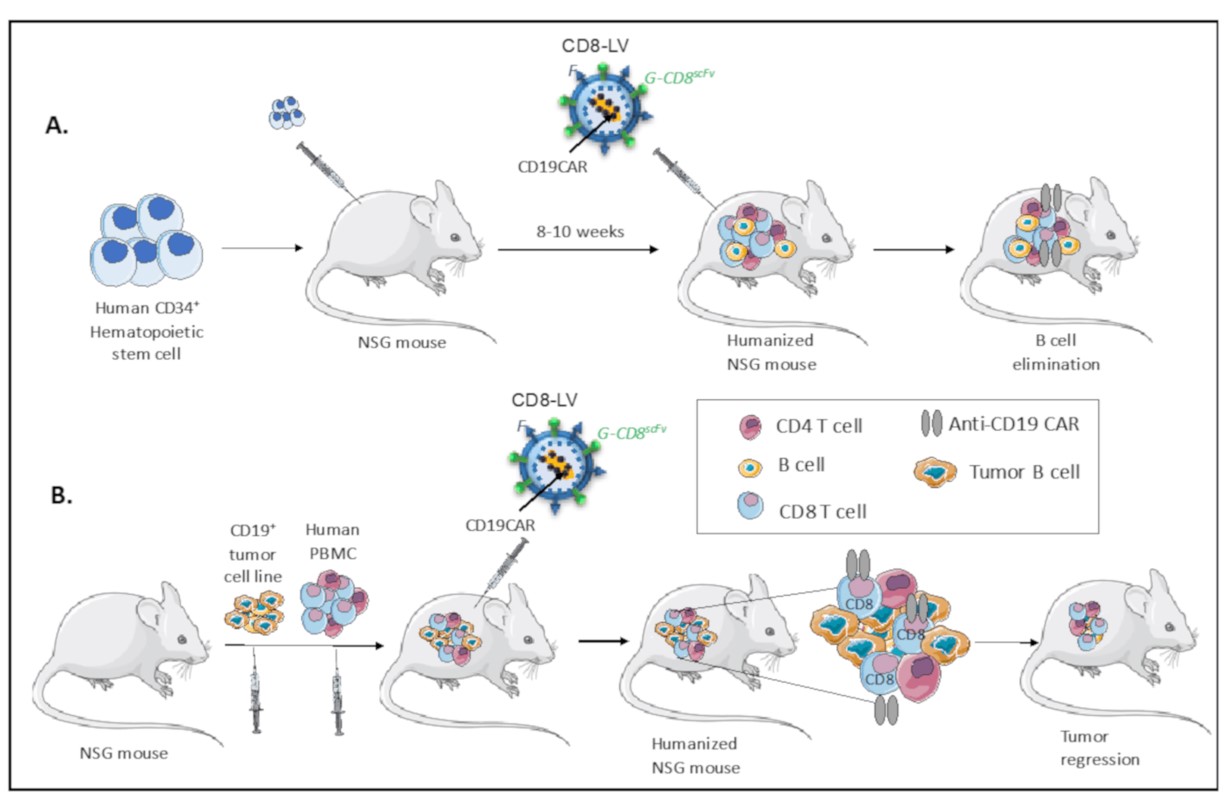
Fig.6 In vivo CAR T cell generation in humanized mice.6
Therapeutic efficacy test of CD19 CAR-T cells in animal models provides valuable information for later clinical application. Creative Biolabs is able to conduct many techniques to evaluate the therapeutic efficacy of the CD19 CAR-T cell therapy including bioluminescence imaging (BLI), survival curve analysis, and FACS analysis of PBMC.
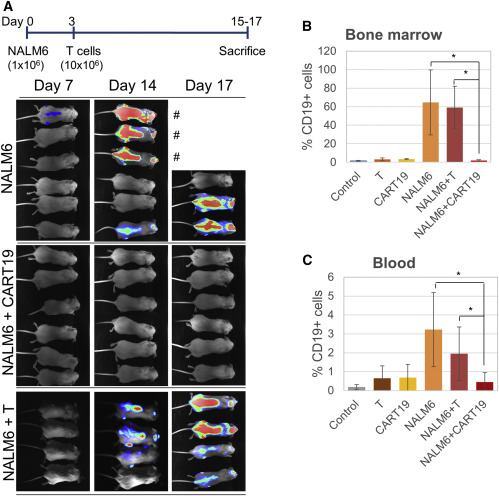
Fig.7 in vivo evaluation of CD19 CAR-T efficacy (Castella, et al., 2018). A) A timeline of experimental design. B) Bioluminescence images showing disease progression on different days.
Toxicity Evaluation CD19 CAR-T
With extensive experience in preclinical in vivo research and project management, Creative Biolabs provides comprehensive CD19 CAR-T cell toxicity evaluation services with appropriate models. The toxicological study services include clinical observation, cytokine release analysis, tumor lysis analysis, tumorigenicity study, and so on.
Please feel free to contact us for more CD19 CAR-T in vivo assays.
References
To support the development of CD19-targeted antibody-based CAR-T therapy, Creative Biolabs has developed a comprehensive series of CD19 molecular-related products, such as CAR vector products, CAR cell products, CAR viral particles, CAR animal cells, and so on. Please browse the list below to find the right product for you.
 Loading...
Loading...
| CAT | Product Name | Target Species | Antibody Clone | Antibody Host | Receptor Construction | Vector Type | Targeting Cell Type | CAR Vector Type | Inquiry & Datasheet |
| CAR-ZP8464 | A uniCAR [Anti-GCN4 CAR], with a Fab-based CAR Adaptor [Anti-CD19 (FMC63)], a Switchable CAR System | Chronic lymphocytic leukemia (CLL) | Lentiviral vector | ||||||
| CAR-ZP8466 | A uniCAR [Anti-FITC CAR], with a Fab-based CAR Adaptor [Anti-CD19 (FMC63)], a Switchable CAR System | Chronic lymphocytic leukemia (CLL) | Lentiviral vector | ||||||
| CAR-ZP8469 | A uniCAR [Anti-GCN4 CAR (28ζ)], with a Fab-based CAR Adaptor [Anti-CD19 (1D3)], a Switchable CAR System | Chronic lymphocytic leukemia (CLL) | Lentiviral vector | ||||||
| CAR-ZP8484 | A uniCAR [Anti-biotin CAR], with a mAb-based CAR Adaptor [Anti-CD19 (Rituximab)], a Switchable CAR System | Chronic lymphocytic leukemia (CLL) | Lentiviral vector | ||||||
| CAR-AB-ZP1 | Anti-FMC63 scFv Monoclonal Antibody, FITC-Conjugated | Human | CAR-T Cell | ||||||
| CAR-AB-ZP2 | Anti-FMC63 scFv Monoclonal Antibody, Biotin-Conjugated | Human | CAR-T Cell | ||||||
| CAR-AB-ZP3 | Anti-FMC63 scFv Monoclonal Antibody, Unconjugated | Human | CAR-T Cell | ||||||
| CAR-ZP8470 | A uniCAR [Anti-GCN4 CAR (BBζ)], with a Fab-based CAR Adaptor [Anti-CD19 (1D3)], a Switchable CAR System | Chronic lymphocytic leukemia (CLL) | Lentiviral vector | ||||||
| CAR-ZP8471 | A uniCAR [Anti-GCN4 CAR(IgG4mTM/mCD28/CD3ζ)], with a Fab-based CAR Adaptor [Anti-CD19 (1D3)], a Switchable CAR System | Chronic lymphocytic leukemia (CLL) | Lentiviral vector | ||||||
| CAR-ZP8472 | A uniCAR [Anti-GCN4 CAR (IgG4mTM/mCD28/m4-1BB/ CD3ζ)], with a Fab-based CAR Adaptor [Anti-CD19 (1D3)], a Switchable CAR System | Chronic lymphocytic leukemia (CLL) | Lentiviral vector | ||||||
| CAR-ZP8473 | A uniCAR [Anti-GCN4 CAR (mCD8TM/m4-1BB/CD3ζ)], with a Fab-based CAR Adaptor [Anti-CD19 (1D3)], a Switchable CAR System | Chronic lymphocytic leukemia (CLL) | Lentiviral vector | ||||||
| CAR-ZP8474 | A uniCAR [Anti-GCN4 CAR (mCD8TM/mCD28/CD3ζ)], with a Fab-based CAR Adaptor [Anti-CD19 (1D3)], a Switchable CAR System | Chronic lymphocytic leukemia (CLL) | Lentiviral vector | ||||||
| CAR-ZP8475 | A uniCAR [Anti-GCN4 CAR (mCD28/m4-1BB/ CD3ζ)], with a Fab-based CAR Adaptor [Anti-CD19 (1D3)], a Switchable CAR System | Chronic lymphocytic leukemia (CLL) | Lentiviral vector | ||||||
| CAR-MV-01LX001 | Anti-CD19 (SJ25C1) h(CD28-CD3ζ) CAR, pMMLV | Human | SJ25C1 | Mouse | scFv-CD28-CD3ζ | Recombinant Moloney murine leukemia virus (MMLV) retroviral vector | T cell | ||
| CAR-MV-01LX002 | Anti-CD19 (FMC63) h(CD28-CD3ζ) CAR, pMMLV | Human | FMC63 | Mouse | scFv-CD28-CD3ζ | Recombinant Moloney murine leukemia virus (MMLV) retroviral vector | T cell | ||
| CAR-MV-01LX003 | Anti-CD19 (HB12a) h(CD28-CD3ζ) CAR, pMMLV | Human | HB12a | Mouse | scFv-CD28-CD3ζ | Recombinant Moloney murine leukemia virus (MMLV) retroviral vector | T cell | ||
| CAR-MV-01LX004 | Anti-CD19 (mR005-2) h(CD28-CD3ζ) CAR, pMMLV | Human | mR005-2 | Mouse | scFv-CD28-CD3ζ | Recombinant Moloney murine leukemia virus (MMLV) retroviral vector | T cell | ||
| CAR-MV-01LX246 | Anti-CD19 (SJ25C1) h(41BB-CD3ζ) CAR, pMMLV | Human | SJ25C1 | Mouse | scFv-41BB-CD3ζ | Recombinant Moloney murine leukemia virus (MMLV) retroviral vector | T cell | ||
| CAR-MV-01LX247 | Anti-CD19 (FMC63) h(41BB-CD3ζ) CAR, pMMLV | Human | FMC63 | Mouse | scFv-41BB-CD3ζ | Recombinant Moloney murine leukemia virus (MMLV) retroviral vector | T cell | ||
| CAR-SB-02LX002 | Anti-CD19 (FMC63) h(CD28-41BB-CD3ζ) CAR, pSBCAR1 | Human | FMC63 | Mouse | scFv-CD28-41BB-CD3ζ | Sleeping Beauty (SB) transposon | T cell | ||
| CAR-SB-02LX243 | Anti-CD19 (SJ25C1) h(CD28-OX40-CD3ζ) CAR, pSBCAR1 | Human | SJ25C1 | Mouse | scFv-CD28-OX40-CD3ζ | Sleeping Beauty (SB) transposon | T cell | ||
| CAR-SB-02LX244 | Anti-CD19 (FMC63) h(CD28-OX40-CD3ζ) CAR, pSBCAR1 | Human | FMC63 | Mouse | scFv-CD28-OX40-CD3ζ | Sleeping Beauty (SB) transposon | T cell | ||
| CAR-SB-02LX245 | Anti-CD19 (HB12a) h(CD28-OX40-CD3ζ) CAR, pSBCAR1 | Human | HB12a | Mouse | scFv-CD28-OX40-CD3ζ | Sleeping Beauty (SB) transposon | T cell | ||
| XS-0722-YF69 | TS-Fluc Anti-Human CD19 scFv (CTL019) 4-1BB-CD3ζ CAR, pCDCAR1 | Human | CTL019 | Mouse | 7H-YB-Fluc-EF1a-scFv-41BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-0722-YF70 | TS-Fluc Anti-Human CD19 scFv (104882) 4-1BB-CD3ζ CAR, pCDCAR1 | Human | 104882 | Human | 7H-YB-Fluc-EF1a-scFv-41BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-0722-YF71 | TS-Fluc Anti-Mouse CD19 scFv (1D3) 4-1BB-CD3ζ CAR, pCDCAR1 | Mouse | 1D3 | Mouse | 7H-YB-Fluc-EF1a-scFv-41BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-0722-YF128 | TS-Fluc Anti-Human CD19 scFv (FMC63) CD28-4-1BB-CD3ζ CAR, pCDCAR1 | Human | FMC63 | Mouse | 7H-YB-Fluc-EF1a-scFv-CD28-41BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-0722-YF129 | TS-Fluc Anti-Human CD19 scFv (CTL019) CD28-4-1BB-CD3ζ CAR, pCDCAR1 | Human | CTL019 | Mouse | 7H-YB-Fluc-EF1a-scFv-CD28-41BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-0722-YF130 | TS-Fluc Anti-Human CD19 scFv (104882) CD28-4-1BB-CD3ζ CAR, pCDCAR1 | Human | 104882 | Human | 7H-YB-Fluc-EF1a-scFv-CD28-41BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-0722-YF131 | TS-Fluc Anti-Mouse CD19 scFv (1D3) CD28-4-1BB-CD3ζ CAR, pCDCAR1 | Mouse | 1D3 | Mouse | 7H-YB-Fluc-EF1a-scFv-CD28-41BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-0722-YF188 | TS-IL15 SA Anti-Human CD19 scFv (FMC63) CD28-CD3ζ CAR, pCDCAR1 | Human | FMC63 | Mouse | 7H-YB-IL15 SA-EF1a-scFv-CD28-CD3ζ | Lentiviral vector | T cell | ||
| XS-0722-YF189 | TS-IL15 SA Anti-Human CD19 scFv (CTL019) CD28-CD3ζ CAR, pCDCAR1 | Human | CTL019 | Mouse | 7H-YB-IL15 SA-EF1a-scFv-CD28-CD3ζ | Lentiviral vector | T cell | ||
| XS-0722-YF190 | TS-IL15 SA Anti-Human CD19 scFv (104882) CD28-CD3ζ CAR, pCDCAR1 | Human | 104882 | Human | 7H-YB-IL15 SA-EF1a-scFv-CD28-CD3ζ | Lentiviral vector | T cell | ||
| XS-0823-LX12 | Anti-hCD19 (12a) ICD(CD28-OX40-CD3ζ) CAR-MA, pAd5f35 Vector | Human | 12a | Adenoviral vectors |
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION