CD35, also known as complement receptor 1 (CR1), plays a crucial role in the complement system. The complement system is part of the immune system that recognizes and removes pathogens, promotes inflammatory responses, and regulates immune function through a series of cascading reactions. CD35, a type I membrane glycoprotein expressed on the surface of erythrocytes and immune cells, is a central regulator of the classical, lectin, and alternative pathways of the complement system, and is a receptor for C3b/C4b complement peptides, which binds the complement C4b and C3b fragments and acts on phagocytes to promote the uptake of particles that activate complement by these cells. It is a cell membrane immunoadhesion receptor that plays a key role in the capture and clearance of complement-conditioned pathogens by erythrocytes and monocytes/macrophages, mediating the binding of these cells to particles of activated complement and immune complexes that remove complement from the circulation.
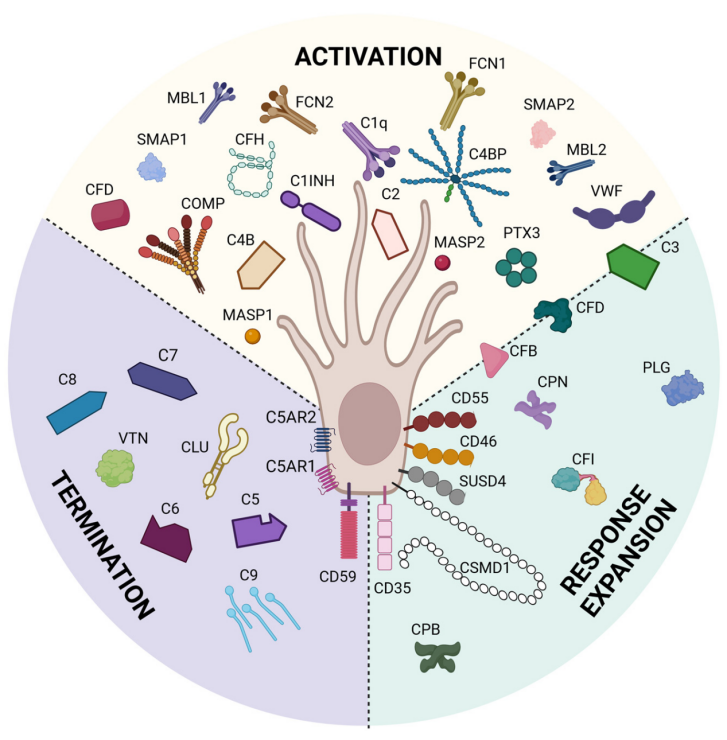
Fig. 1 Cells express complement proteins.1
CD35 Composition and Distribution
CD35 exists in the following forms: a membrane-bound form, which functions on the cell surface and is involved in the regulation of the complement system, helping to clear immune complexes and C3b/C4b-encapsulated particles from plasma, as well as regulating the inflammatory response. Another form is the non-membrane-bound soluble form of CR1 (sCR1) found in plasma, which is produced by proteolytic cleavage and released into circulation from leukocytes (especially polymorphonuclear leukocytes). It has an immune function in body fluids, binds to complement fragments, and may be involved in the regulation of local immune responses and inflammatory processes. Levels of soluble CD35 may change in certain disease states, making it a potential biomarker for certain pathologies.
Distribution of CD35: CD35 is mainly found in erythrocytes, CD4+ T cell subsets, mature B cells, follicular dendritic cells, monocytes, and eosinophils.
For CD35/CR1 research, we can offer the following related products:
CD35 Structure
CD35 is a transmembrane protein. The extracellular domain of CD35 consists of 30 short complement regulatory (SCR) structural domains, also known as short consensus repeats, Sushi or complement control protein structural domains. Each SCR structural domain contains 61 amino acid residues with two conserved disulfide bonds and a buried conserved tryptophan residue. Of the CR1 sequences, the first 28 are organized into four long homologous repeat sequences (LHRs), each with seven contiguous SCRs. The functional domains of the CR1 proteins are specifically distributed among the different LHRs. The LHR-A region of CD35 binds predominantly to C4b, whereas the LHR-B and LHR-C regions bind to C3b/C4b and PfEMP1 and have cofactor activity for cofactor I-mediated cleavage of C3b and C4b. The LHR-D region has binding sites for mannose-binding lectin (MBL) and C1q. SCR-25 has binding sites for the Swain-Langley (Sl) and McCoy (McC) Knops blood group antigens in LHR-D. The LHR-D region also has binding sites for the C3b/C4b antigen. Soluble CD35 (sCR1) contains 30 short consensus repeat (SCR) structural domains that are identical to the extracellular domains of CR1, but because sCR1 lacks transmembrane and cytoplasmic tails, it is structurally different from CR1 immobilized on the cell surface.

Fig. 2 The structure, genetic polymorphisms, expression ofCR1/CD35.2
CD35 Signaling Pathway and Regulation
CD35 is involved in regulatory T cell activation and proliferation: binding of CD35 enhances IL-10 production and reduces IFNγ production, contributing to the transformation of CD4+ T cells into a regulatory T cell (Treg) phenotype. Additionally, the co-binding of CD35 and CD46 synergistically enhances the proliferation of CD4+ T cells. Furthermore, the combination of CD35 and CD46 enhances granzyme B expression in activated CD4+ T cells. Overall, CD35 plays an important role in regulating the function and phenotypic transformation of CD4+ T cells.
CD35 is also involved in the regulation of complement system activation: it regulates the degradation-accelerating activity (DAA) of C3 and C5 convertases of the classical and alternative pathways through its different structural domains (e.g., LHR-A, LHR-B, LHR-C). Different structural domains of CR1 contribute differently to the DAA of C3 and C5 convertases of the classical pathway. For example, for the C3 convertase of the classical pathway, the LHR-A structural domain plays a major role in DAA, while the LHR-B and LHR-C structural domains contribute to a lesser extent. In contrast, for the C5 convertase of the classical pathway, the LHR-A, LHR-B, and LHR-C structural domains are all required, acting synergistically to provide complete activity. Additionally, CR1 exerts cofactor activity (CFA) by binding to C3b and C4b and facilitating cleavage of C3b and C4b by complement factor I. CR1 has more cleavage activity on C3b than on C4b, correlating with its binding affinity for C3b and C4b. Thus, CR1 regulates the activities of C3 and C5 convertases of the classical and alternative pathways through the action of its different structural domains, participating in the regulatory mechanism of the signaling pathway.
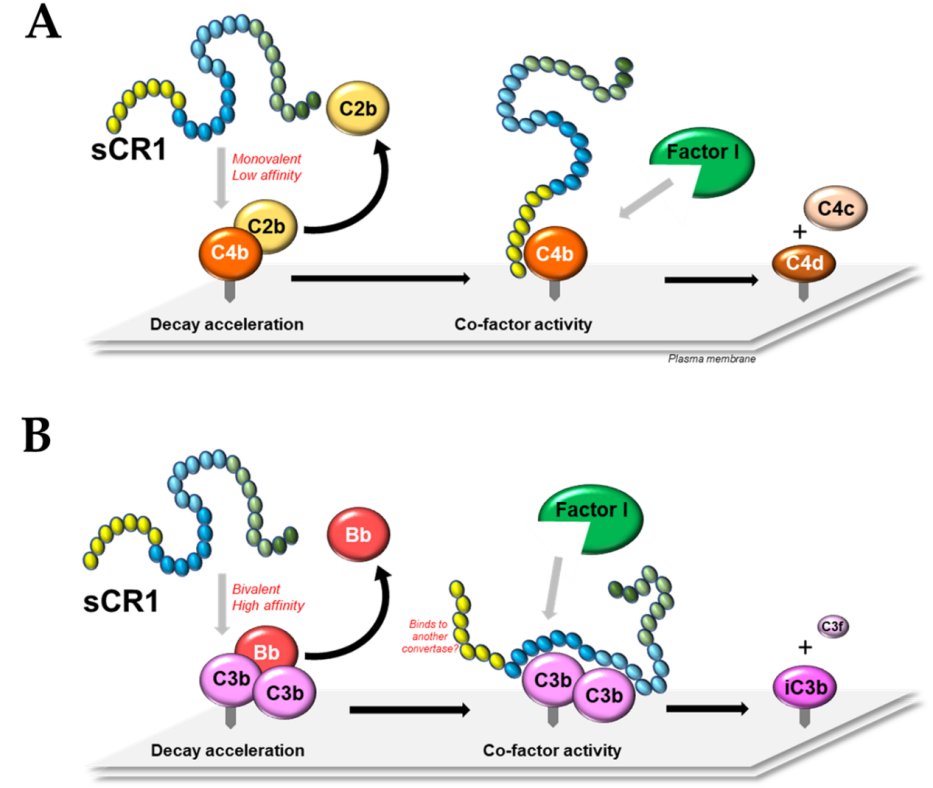
Fig. 3 A model of the molecular mechanisms of soluble CD35.3
CD35 Clinical Value
Diagnosis: The expression level of CD35 can be used as a diagnostic marker for certain hematologic diseases, especially in differentiating chronic lymphocytic leukemia (CLL) from other B-chronic lymphoproliferative disorders (B-CLPDs). Studies have shown that low expression of CD35 correlates significantly with the diagnosis of CLL, especially in the differential diagnosis with other B-CLPDs such as MCL.
Disease monitoring: Changes in CD35 expression may reflect disease progression or response to therapy. In some cases, changes in CD35 levels may be used to monitor a patient’s response to therapy, particularly in immunotherapy or complement therapies.
Immunomodulation: CD35 plays a key role in regulating the activation of the complement system and suppressing inflammatory responses. It is involved in regulating the activation of the complement system by binding to the complement fragment C3b/C4b and plays a role in clearing immune complexes and modulating the inflammatory response in particular.
Therapeutic target: Due to its role in a variety of diseases, CD35 may be a potential target for therapy. Regulating the complement activity by targeting CD35 may provide new avenues for the treatment of certain inflammatory and autoimmune diseases.
In conclusion, CD35, as an important complement receptor, plays a key role in the development of many diseases, and its in-depth study can help understand disease mechanisms and provide new strategies for clinical treatment.
References:
- Washburn, Rachel L., and Jannette M. Dufour. “Complementing testicular immune regulation: the relationship between sertoli cells, complement, and the immune response.” International Journal of Molecular Sciences4 (2023): 3371.
- Liu, Dong, and Zhong-Xiang Niu. “The structure, genetic polymorphisms, expression and biological functions of complement receptor type 1 (CR1/CD35).” Immunopharmacology and immunotoxicology4 (2009): 524-535.
- Hardy, Matthew P., et al. “The Molecular Mechanisms of Complement Receptor 1—It Is Complicated.” Biomolecules 13.10 (2023): 1522.
