Creative Biolabs is always dedicated to providing the most high-quality and customized drug discovery services for our worldwide customers based on years of experience and high-end technologies.
What is Complement C5 Convertase?
It is well known that the complement system is essentially a cascade amplification response, which functions as a “complement” of the immune system playing an important role in immune response, pathogen clearance, and inflammation. The complement cascade won’t be activated without the catalysis of several enzymes, among which the most important ones are C3 convertase and C5 convertase.
C5 convertase is a kind of serine protease that cleaves the complement component C5 (C5) into fragments C5a, an anaphylatoxin and a chemotactic factor, and C5b, the first component of the membrane attack complex (MAC). Two different C5 convertases respectively mediate the classical pathway/lectin pathway and the alternative pathway in the complement system. The other two uncommon C5 convertases also have been reported in the forms of fluid phase: the cobra venom factor-dependent C5 convertase (CVFBb) and the classical pathway enzyme C4b2boxy3b.
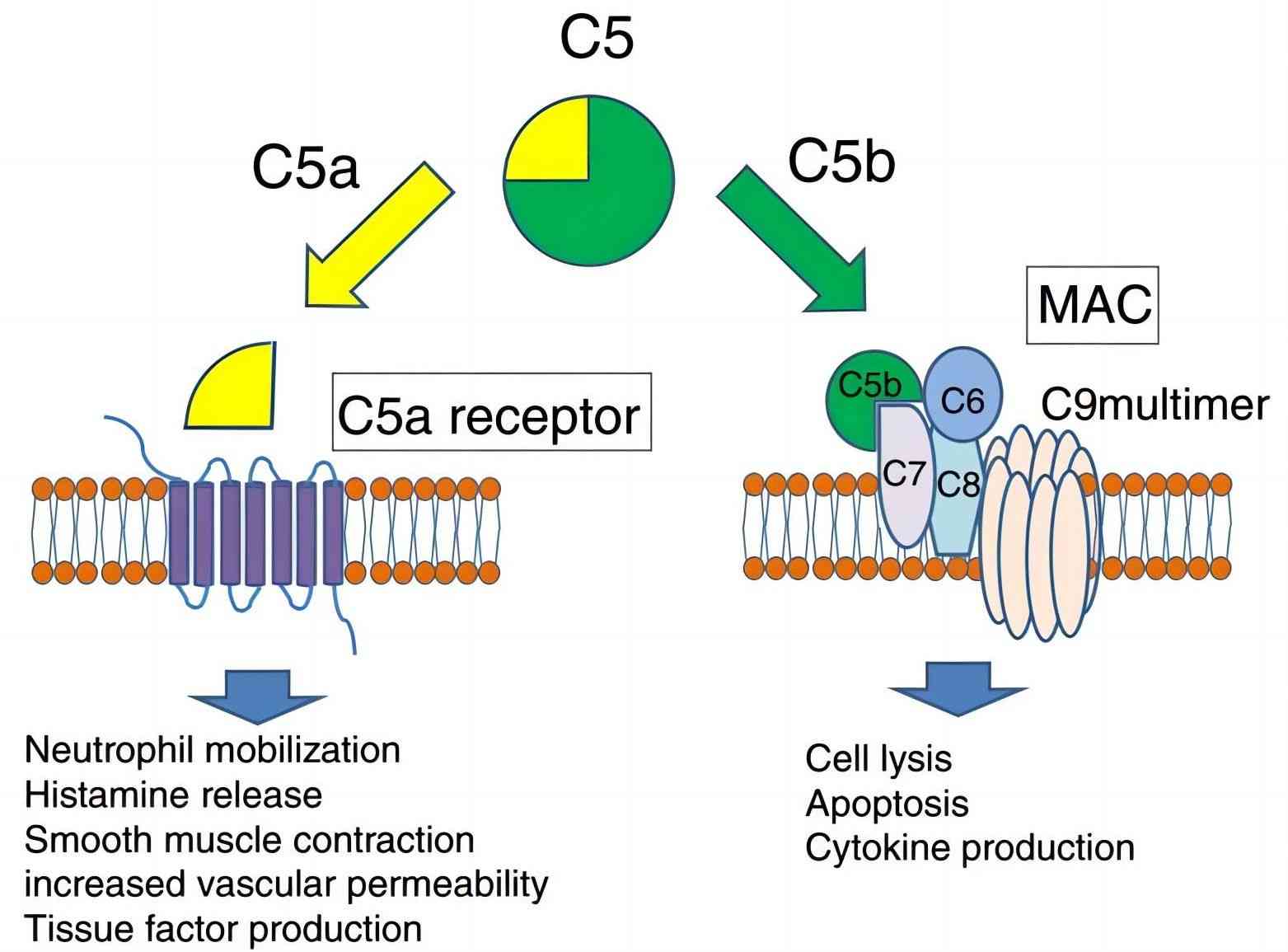
Fig. 1 C5 convertase cleaves C5 into C5a and C5b.1, 3
Functions and Structures of C5 Convertases
No matter in complement classical pathway or lectin pathway and alternative pathway, the C5 convertase functions to selectively cleaves the C5 into C5a and C5b at position 74-75 in the C5 α-chain, which is the last enzymatic step of the complement cascade activation. Afterward, the C5a mainly mediates chemotaxis, inflammation, and immune response, whereas, the C5b involves the formation of MAC in the terminal complement cascade. Despite the identical functions of C5 convertase shares in the three pathways, the structures differ slightly in these pathways.
-
C5 convertase in complement classical pathway
Once the classical pathway is activated, C2 and C4 are respectively cleaved by C1 complex into C2a and C2b, C4a and C4b, of which C2b and C4b form the classical pathway C3 convertase (C4bC2b), which converts C3 into C3a and C3b. Then the classical pathway C5 convertase is formed by complement fragments C2b, C4b, and C3b, which is C4b2b3b.
-
C5 convertase in the complement lectin pathway:
Although the activation and C2b/C4b formation in the lectin pathway is different from that in the classical pathway, the lectin pathway C5 convertase structure is the same as the classical pathway C5 convertase.
-
C5 convertase in the complement alternative pathway:
The alternative pathway C5 convertase formation starts from the spontaneous cleavage of C3. When the alternative pathway is auto-activated, a little C3 conformation alters and is hydrolyzed generating C3a and C3b. The C3b binds to Bb, a fragment generated from the cleavage of complement factor B (CFB) by the complement factor D (CFD), forming alternative pathway C3 convertase (C3bBb). The alternative pathway C5 convertase is formed when an additional C3b binding to C3bBb, which is C3bBbC3b.
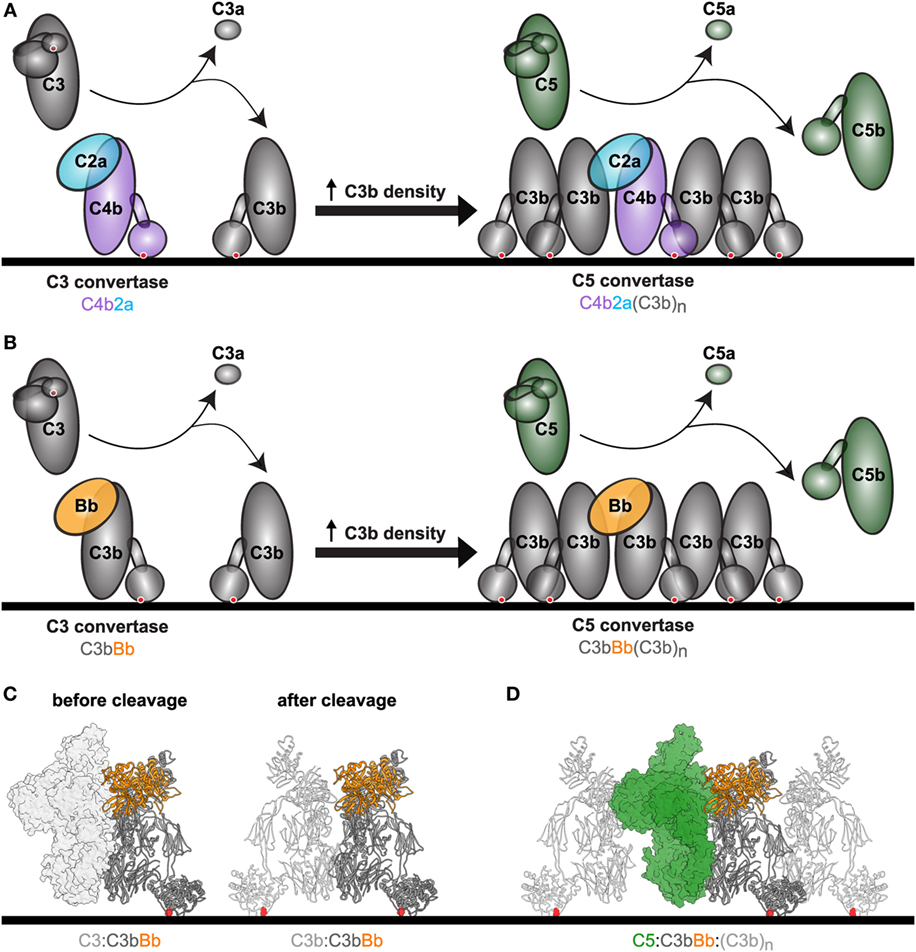
Fig. 2 C5 convertase cleaves C5 into C5a and C5b.2, 3
Our Services
As one of the essential convertase enzymes, the C5 convertase associates with the activity of the whole complement system. The abnormality of C5 convertase is closely related to a series of diseases, such as hemolytic uremic syndrome (HUS) caused by reduced blocking of C5 convertase, inflammation, and autoimmune diseases. The C5 convertase is a potential complement therapeutic target in the related disease treatment. As an expert in complement therapy development, Creative Biolabs provides custom solutions and related products for the C5 convertase. Our services mainly include verification of C5 convertase activity, C5 convertase deficiency identification, and C5 convertase inhibitor development.
For the specific question about the C5 convertase in the complement system, you can directly contact us or discuss it with our scientists. Our professional staff will provide the most tailored solutions for your projects.
Published Data
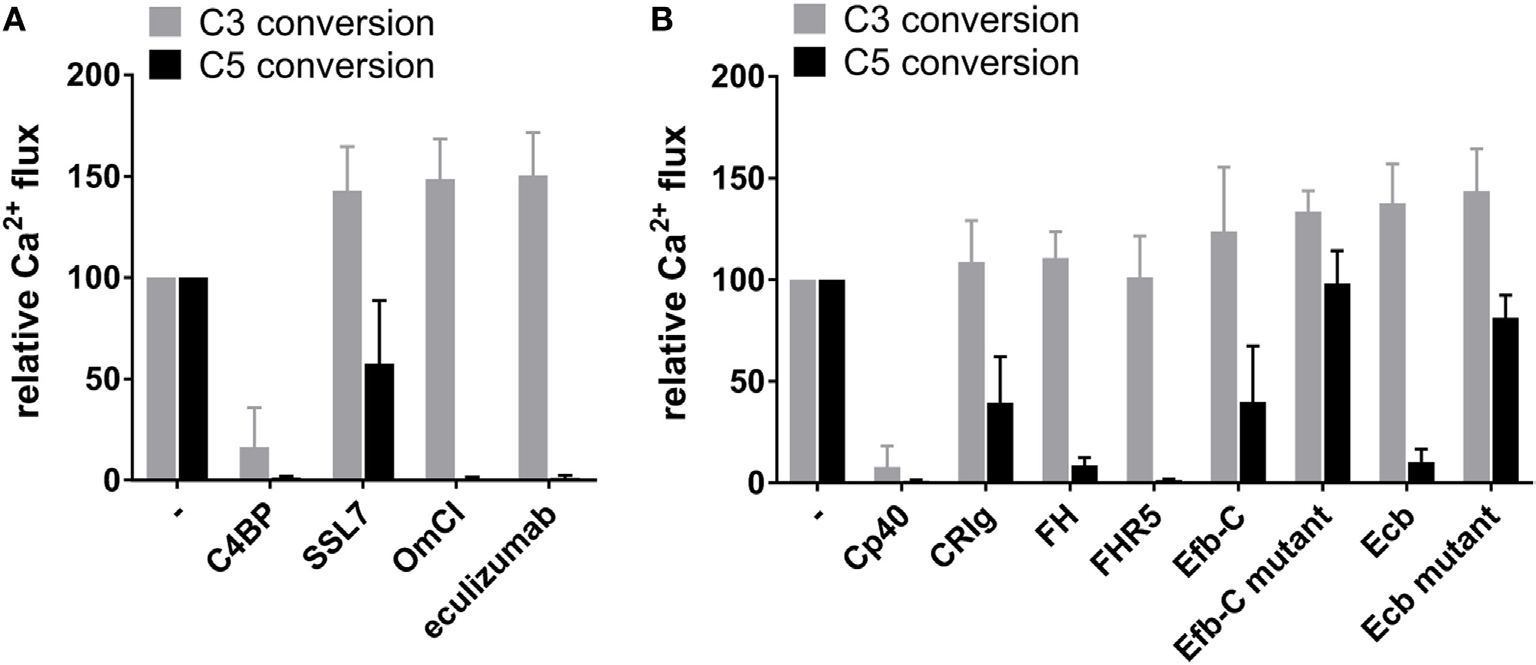 Fig.3 Inhibitors impact on C3 and C5 conversion in the classical pathway.2, 3
Fig.3 Inhibitors impact on C3 and C5 conversion in the classical pathway.2, 3
Researchers have established a system to generate purified C5 convertases of the alternative pathway on beads coated with C3b, enabling the quantification of C5 conversion by analyzing the release of C5a. They investigated the influence of C3b- and C5-binding molecules within the classical pathway convertase framework, revealing that C4BP effectively hindered both C3 and C5 conversion. Intriguingly, certain C5-binding molecules selectively impeded C5 conversion while sparing C3 processes. Moreover, findings showed that proteins like FHR5, Efb-C, and Ecb had similar inhibitory effects on both alternative pathway and classical pathway pathways, indicating a uniform role of accessory C3b. These discoveries pave the way for developing specific convertase inhibitors targeting complement-related conditions.
Related Product
References
-
Horiuchi, Takahiko, and Hiroshi Tsukamoto. "Complement-targeted therapy: development of C5-and C5a-targeted inhibition." Inflammation and regeneration 36 (2016): 1-5.
-
Zwarthoff, Seline A., et al. "Functional characterization of alternative and classical pathway C3/C5 convertase activity and inhibition using purified models." Frontiers in immunology 9 (2018): 1691.
-
Distributed under Open Access license CC BY 4.0, without modification.
Questions & Answer
A: Emerging therapeutic approaches targeting C5 convertases include monoclonal antibodies, which inhibit C5 cleavage, and small molecules that target the formation or stabilization of C5 convertase complexes. Challenges in this field include optimizing the specificity and duration of inhibition to avoid compromising host defense against infections while effectively mitigating complement-mediated diseases.
A: Yes, C5 convertases are pivotal for host defense against pathogens as they initiate the formation of MAC and the release of C5a, which have distinct roles in immunity. The MAC, assembled on the pathogen's surface, can form pores, leading to cell lysis. Meanwhile, C5a acts as an anaphylatoxin, recruiting immune cells like neutrophils and monocytes to the site of infection. This immune cell recruitment and enhanced phagocytosis are crucial for the efficient clearance of pathogens.
A: Recent research has shed light on the regulatory mechanisms of C5 convertases, revealing C5 convertases as novel targets for therapeutic intervention. For instance, the discovery of molecules that modulate the stability or activity of C5 convertases could provide new avenues for precise complement regulation. Additionally, advances in structural biology and computational modeling have enhanced our understanding of C5 convertase assembly and function, offering opportunities for rational drug design in this area.
For Research Use Only.
Related Sections:



 Fig.3 Inhibitors impact on C3 and C5 conversion in the classical pathway.2, 3
Fig.3 Inhibitors impact on C3 and C5 conversion in the classical pathway.2, 3