Structure of Complement Regulatory Protein Factor H
Complement Factor H, also known as Factor H and CFH, is a sialic acid-containing glycoprotein that plays an integral role in regulating the complement-mediated immune system. The human CFH gene is located in the region of the complement activation gene cluster where the regulatory factors reside. It contains 25 exons and 24 introns, 94 kb and 91.4 kb bases long, respectively, encoding a total of 1,231 amino acids. The CFH gene encodes Factor H protein, a Factor H-like protein 1 (FHL-1), and five Factor H-associated proteins (FHR1-5) with a molecular weight of 155 kDa. Factor H proteins belong to the β1H globulins. They are the most characterized members of the Factor H protein family and serve as important negative regulators of alternative complement pathway activity. Factor H proteins contain several structural domains that interact with ligands such as C3, C3b, heparin, and C-reactive protein (CRP), with three binding sites for C3b and three for heparin. CFH protects its own cells from complement activation but not bacteria and viruses. Misregulation of CFH may adversely affect the ability to deal with foreign infections or to reduce the complement activity of the host cell, leading to various effects.
Creative Biolabs is dedicated to providing monoclonal antibodies, polyclonal antibodies, recombinant proteins, ELISA kits, etc., for CFH research. Example:
- Sheep anti-human complement factor H polyclonal antibody
- Pig complement factor H ELISA kit
- Human complement factor H autoantibody ELISA kit
- Complement factor H-related protein 1
- Complement factor H-related protein 2
Mutations in the Factor H gene have been associated with a variety of serious diseases, including rare kidney diseases (HUS) and membranoproliferative glomerulonephritis (MPGN), as well as the more common age-related macular degeneration (AMD). Structure determines function, and the complex physiology of CFH underlies the complex diseases that result.
Function of Complement Regulatory Protein Factor H
CFH is a multistructural and multifunctional plasma glycoprotein present in normal serum, whose primary role is to bind to host surfaces to prevent them from being activated by complement. CFH stabilizes complement activity in the blood at a concentration of 400-800 µg/ml, thereby targeting the immune response. It binds to C3b, accelerating the breakdown of the alternative pathway C3 convertase and helping to inactivate C3b, thereby protecting the body from unwanted tissue damage. This plasma protein is mainly produced by the liver, and small amounts of CFH can also be produced by other types of cells, such as retinal pigment epithelial cells, peripheral blood lymphocytes, fibroblasts, neurons, and glial cells.
Factor H and/or FHL-1 are important negative regulators in the complement paracrine activation pathway, which starts to function at the early stage of the immune response. Therefore, CFH plays a crucial role in inhibiting early complement activation, inhibiting the formation of the complement paracrine C3 converting enzyme, thereby inhibiting the activation of the complement paracrine pathway on the surface of circulating cells, and attenuating the subsequent lysogenic effect and inflammatory response. Specifically:
- CFH protein binds complement C3b and inhibits the formation of the C3 converting enzyme of the bypass pathway.
- It displaces C3b from the formed C3 converting enzyme of the bypass pathway, accelerates the degradation of the C3 converting enzyme, and interferes with the generation and stabilization of the C3 converting enzyme of the complement bypass pathway.
- In addition, there is also a binding site on CFH for CRP, which plays a role in the negative regulation of inflammation
- Meanwhile, CFH protein, as an important effector of innate immunity, recognizes self and foreign bodies and plays an important role in innate immunity.
Complement Regulatory Protein Factor H and COVID-19
COVID-19 may cause long-lasting sequelae, so research on its pathogenesis remains a topic of high interest. The clinicopathologic manifestations of COVID-19 are consistent with an innate immune host response. Hyperactivation of the blood-borne complement system is a major component of innate immunity and plays a key role not only in inflammatory syndromes but also in the microvascular and macrovascular thrombosis that usually occurs. Several soluble and membrane-anchored proteins, including CFH, prevent excessive complement activation. Reduced functional expression of one or more of these negative regulators increases the risk of atypical hemolytic uremic syndrome (aHUS) with microvascular thrombosis, inflammation, and multiorgan damage, a syndrome with characteristics similar to COVID-19.
Literature indicates that data from screening by the complement system showed that AP was activated in all COVID-19 patients, and serum CFH levels were significantly lower in COVID-19 patients compared to healthy controls. Thus, reduced functional levels of the negative AP regulator FH led to overactivation of the AP pathway, C3a generation, and C3 fragmentation deposition, and a significant increase in C5a serum levels, as well as providing the idea that inhibition of C3 and C5 could be used to treat patients.
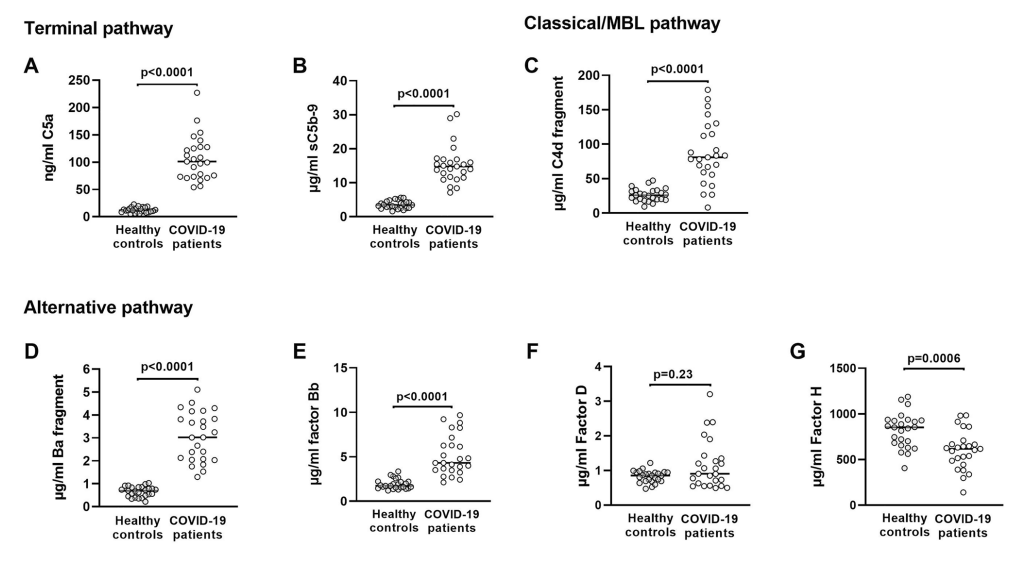
Fig. 1 Markers of complement pathway activation or complement proteins in serum from healthy general population controls and critically ill COVID-19 patients admitted to the ICU.1
Complement Regulatory Protein Factor H and AMD
AMD is a senile alteration of macular structures. The main manifestation is that the retinal pigment epithelial cells have decreased phagocytosis and digestion of the disc membrane of the outer segment of the optic cells. As a result, the residual vesicles of the disc membrane that have not been completely digested are retained in the cellular plasma of the base and are discharged to the outside of the cell, and are deposited in the Bruch’s membrane, forming vitreous membrane warts. Such changes are more pronounced due to the structural and functional peculiarities of the macula. Vitreous warts are also seen in elderly people with normal vision, but the resulting pathological changes lead to macular degeneration.
Polymorphisms in FH have been identified in genome-wide association studies as a key susceptibility factor for AMD, and binding of CFH to CR3 receptor inhibits the activation of the integrin-related receptor CD47 by platelet-responsive protein-1, which is necessary for the homeostatic elimination of subretinal phagocytes. This inhibition is potentiated by the AMD-associated CFH variant H402Y, which results in the pathological accumulation of subretinal phagocytes.
Complement Regulatory Protein Factor H and Atherosclerosis
Atherosclerosis (AS) is a major cause of coronary heart disease, cerebral infarction, and peripheral vascular disease. Impaired lipid metabolism is the basis of atherosclerosis. Because of the yellow, atheromatous appearance of the lipids that accumulate in the lining of the arteries, it is called atherosclerosis. Over the past decades, it has been found that activation of the bypass pathway of the complement system produces a large number of unstable C3 converting enzymes, as well as a variety of inflammatory mediators produced during complement activation, such as C5a and complement C5b-9, which cause damage to vascular endothelial cells, aggregation of inflammatory mediators, platelet aggregation, and hyperplasia of vascular smooth muscle cells, affecting the development of atherosclerosis. Compared with normal arteries, C3b/iC3b and C5b-9 deposition were significantly increased in arteries with atherosclerotic lesions. This is, of course, very much related to the reduction of CFH, which leads to the overactivation of the complement system.
This demonstrates that CFH, as an important negative regulator of the complement system, plays an important regulatory role in preventing atherosclerosis. CFH accelerates the inactivation of the C3 converting enzyme in the bypass pathway by binding to C3b in the artery’s endothelium, reduces the generation of the end-product of complement activation, C5b-9, and inhibits complement activation in the superficial layer of the artery’s endothelium, thus slowing down the development of atherosclerotic plaques.
Reference:
- Leatherdale, Alexander, et al. “Persistently elevated complement alternative pathway biomarkers in COVID-19 correlate with hypoxemia and predict in-hospital mortality.” Medical Microbiology and Immunology (2022): 1-12.
