The complement system plays a crucial role in the body’s defense against harmful invaders. It is an essential component of the innate immune response, and its end products, C3a and C5a, exert their physiological and pathological responses primarily through two G protein-coupled receptors, C3aR and C5aR1. However, the molecular mechanisms underlying the recognition, activation, and signaling bias of these receptors remain largely unknown.
In a new study, researchers from USC, India, Australia, and Switzerland have shed light on these proteins’ mysteries. Their research has the potential to pave the way for innovative treatments for various diseases, including severe COVID-19, rheumatoid arthritis, neurodegenerative diseases, and cancer.
The complement cascade lies at the core of our immune response, a series of events triggered when a potential threat is detected. This process produces C3a and C5a, which activate specific receptors on the cell surface, initiating internal signals. The exact mechanism of these receptors, particularly C5aR1, has always been a mystery.
Using advanced cryo-electron microscopy (cryo-EM) techniques, the authors of this study have provided nine cryo-EM structures of C3aR and C5aR1, activated by natural and synthetic agonists. These structures reveal different binding pocket topologies fo complement allergenic toxins, providing valuable insights into receptor activation and signal transduction coupling. The author also elucidates the structural basis of naturally occurring inhibition of the C5a inflammatory response through protein hydrolysis-mediated cleavage of terminal arginine, as well as the G protein signaling bias induced by a C3aR peptide agonist. Their study sheds light on the internal structure of complement allergy toxin receptors and contributes to structure-driven drug discovery for targeting these receptors in a range of diseases.
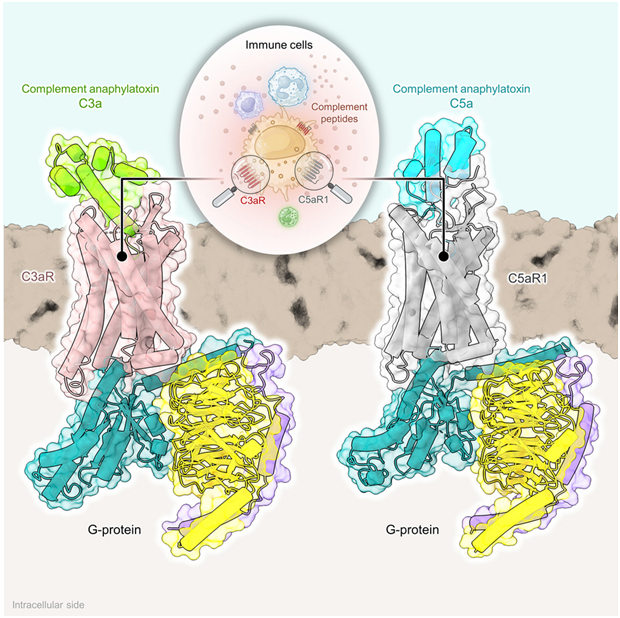
Fig. 1 Inner workings of C3aR and C5aR1.1
As the global community continues to grapple with diseases that affect millions of people, understanding the intricacies of the immune system becomes increasingly essential. This study enhances a deeper understanding of this issue and sets the stage for future research aimed at harnessing the body’s natural defenses.
Reference:
- Yadav, Manish K., et al. “Molecular basis of anaphylatoxin binding, activation, and signaling bias at complement receptors.” Cell 186.22 (2023): 4956-4973.
