The blood-brain barrier (BBB) poses a significant challenge to the systemic delivery of mRNA to diseased neurons.While extracellular vesicles (EVs) derived from leukocytes can traverse the BBB, efficiently loading lengthy mRNAs into EVs and improving their uptake by neurons remains problematic. A recent collaboration between Cornell University and the Massachusetts Institute of Technology (MIT) has addressed this issue, as outlined in an article published in the journal Nature Biomedical Engineering. Their approach involves a method to enhance mRNA loading and neuronal uptake by engineering leukocytes to produce EVs containing retrovirus-like mRNA packaging shells.
Systemic drug delivery to neurons is limited by the BBB, as well as by low uptake rates of vectors and inefficient release onto neurons. Most biologics, including recombinant proteins, therapeutic antibodies, and nucleic acids, are unable to penetrate the BBB. Adeno-associated viruses (AAVs) can deliver DNA to neurons, while recombinant proteins and therapeutic antibodies can cross the BBB via receptor-mediated transporters specific to certain peptides, such as insulin or transferrin. Messenger RNA (mRNA) has emerged as a promising therapeutic agent for preventing and treating diseases. However, for mRNA to be effective in the body, it requires a safe, efficient, and stable delivery system that shields it from degradation and facilitates cellular uptake and release. Various viral and nonviral vectors have been developed for this purpose, including retroviral vectors, lipid nanoparticles, polymers, protein derivatives, and membrane-enclosed vesicles. However, achieving efficient and targeted delivery of RNA therapeutics in vivo remains a formidable challenge.
Inflammatory stimulation of the brain leads to the destruction of the BBB. Brain microvascular endothelial cells (BMECs) exhibit increased permeability, accompanied by an upregulation of leukocyte adhesion molecules. This facilitates the entry of circulating leukocyte-derived extracellular vesicles (EVs) into the brain, rendering them excellent candidates for neuron-targeted drug delivery. The permeability of the BBB to leukocyte EVs is heightened in various neurological diseases, including age-related chronic inflammation, neurodegenerative diseases, and more severe pathological conditions such as systemic inflammation and secondary injuries (e.g., stroke). However, the clinical translation of EV-based therapeutics for these diseases is limited by low payload encapsulation efficiency and the inability to control the molecules loaded into EVs from donor cells. The payload of EVs may comprise proteins, DNA, RNA, lipids, nutrients, and metabolic waste products. The exclusion of unnecessary cellular components from EVs is challenging, impacting both loading capacity and the potential delivery of harmful components to the target.
Various systems have been developed for loading small RNAs (such as siRNA and miRNA) into EVs, but efficiently enriching of long mRNAs into EVs remains a challenge. Extremely low copy numbers of endogenous EV-associated RNAs have been reported, ranging from 0.02 to 1 RNA per EV. Small RNAs are more efficiently packaged into EVs compared to long mRNAs (0.01 to 1 miRNA vs. 0.001 intact long RNAs per EV). Only 8% of donor cells exhibited detectable mRNA in their EVs. Previous methods for loading mRNA into EVs include passive and active encapsulation. For example, by incubating with macrophage-derived EVs following ultrasound and extrusion, or treating with saponins to address Parkinson’s disease. However, this post-EV isolation and loading approach has limited capacity as EVs already filled with donor cell components cannot be unloaded to accommodate drugs. Alternatively, EVs can be engineered at the mother cell level. The efficient and selective incorporation of mRNA into EVs under natural conditions is crucial for designing EVs as therapeutic drug carriers.
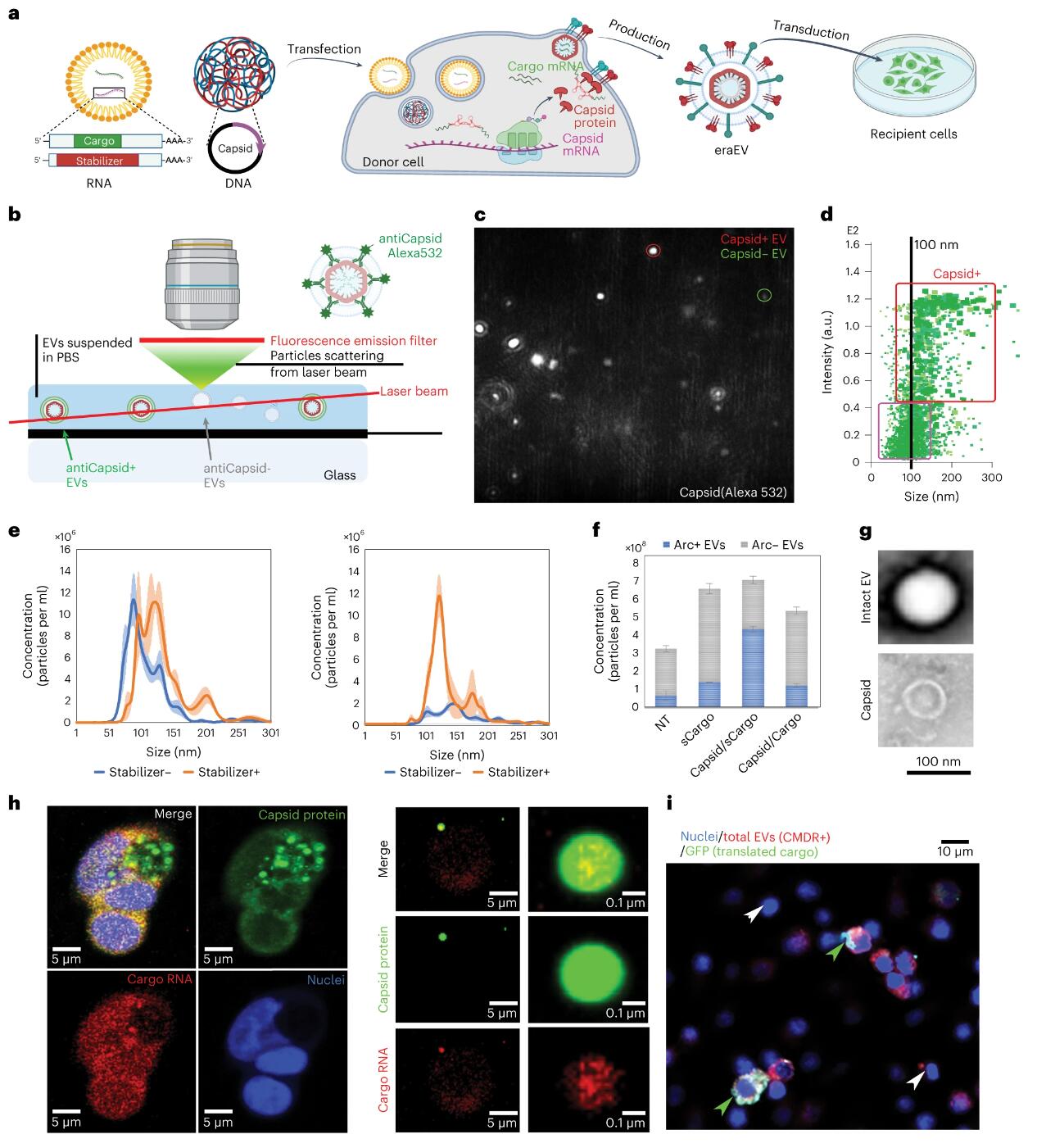
To enhance the loading of RNA payloads into EVs, the authors introduced retrovirus-like protein shells into the EV lumen. These protein shells naturally occur in the human brain and play a role in facilitating intercellular communication within the central nervous system. They originate from transposable genomic elements spanning eukaryotic domains, known as long terminal repeat (LTR) retrotransposons, which share an evolutionary origin with retroviruses. LTR retroviruses, along with the retrotransposon coat protein (Gag) homolog Arc (activity-regulated cytoskeleton-associated protein), may have evolved in parallel and function similarly to infectious RNA retroviruses. Another Gag homolog, PEG10, was recently modified to form virus-like particles for in vitro mRNA delivery. The Arc protein self-assembles into a virus-like shell to encapsulate mRNA, which is then released from neurons and delivered to receiving neurons via receptor-mediated endocytosis. Although Arc EVs hold potential as drug delivery vectors, their use in drug delivery remains incompletely studied. Arc and Gag are reported to be inefficient at mRNA transduction and less specific for payloads without the addition of their untranslated regions (UTRs). In this study, an Arc 5’ UTR (A5U) was included in the payload build to stabilize the shell and increase payload carrying capacity. Therefore, the authors introduced the Arc protein coat into EVs and added the A5U RNA motif stabilizer to achieve effective mRNA encapsulation and delivery.
In addition to enhanced payload-carrying capabilities, the system offers several other advantages. First, engineered retrotransposon Arc extracellular vesicles (eraEVS) produced by autologous leukocytes are immunologically inert and accumulate in the inflammatory microenvironment, crossing the blood-brain barrier with the assistance of membrane molecules from donor leukocytes. Furthermore, the Arc component recruits wrapping proteins during self-assembly, promoting the cellular uptake of EVs through its natural function in mediating molecular exchange between neurons. Moreover, the virus-like shell renders eraEVs more stable than other engineered RNA-loaded EVs, protecting the payload from nuclease degradation until its release is triggered. Besides leveraging these unique virus-like properties, eraEVs are safe, serving as short-lived drug carriers incapable of replication, infection, or genetic information insertion into the recipient’s genome. Crucially, relying on the natural targeting capabilities of EVs across different cell types, eraEVs can be generated from various donor cells and applied in diverse biomedical contexts. This study highlights an endogenous virus-like system capable of loading and delivering mRNA in vivo, specifically targeting diseased neurons through systemic administration.
 Enhanced stability of Arc EV via A5U motif
Enhanced stability of Arc EV via A5U motif
The study transfected immortalized and primary bone marrow-derived leukocytes with DNA or RNA encoding the activity-regulated cytoskeleton-associated protein (Arc) of coat formation and the RNA element of the Arc 5′ untranslated region that stabilizes the coat. These engineered EVs inherit the endothelial adhesion molecules of donor leukocytes, recruit endogenous packaging proteins to their surface, cross the BBB, and enter sites of neuroinflammation in neurons. These EVs generated from autologous donor leukocytes are immunologically inert and enhance neuronal uptake of packaged mRNA in a mouse model of low-grade chronic neuroinflammation.
Reference:
Gu W, Luozhong S, Cai S, et al. Extracellular vesicles incorporating retrovirus-like capsids for the enhanced packaging and systemic delivery of mRNA into neurons. Nat Biomed Eng. Published online February 19, 2024. doi:10.1038/s41551-023-01150-x
Related Services:
Brain-Targeted Exosome Modification Service
