Extracellular vesicle (EV)-based therapeutic approaches still pose challenges in clinical application due to their rapid clearance, limited residence, and low yield. Although hydrogels possess the ability to impede physiological clearance and increase regional retention, they often fail to efficiently release the bound EVs, resulting in reduced accessibility and bioavailability. Recently, scientists published an article in Adv Mater reporting on an intelligent hydrogel where the release of EVs is regulated by proteins on the EV membrane. By utilizing EV membrane enzymes to promote hydrogel degradation, sustained retention and self-stimulated EV release can be achieved at the administration site. To accomplish this goal, membrane proteins with matrix-degrading activity in extracellular vesicles derived from mesenchymal stem cells (MSC-EVs) were identified using comparative proteomics. Subsequently, a hydrogel composed of self-assembling peptides that was quickly degraded by membrane enzymes present in MSC-EVs was designed and synthesized. After intranasal administration, this peptide hydrogel can promote sustained and thermosensitive release of MSC-EVs, thereby prolonging the retention time of MSC-EVs and significantly enhancing their potential to treat Alzheimer’s disease.

Extracellular vesicles (EVs) are cell-derived structures that carry biologically active molecules, including proteins, mRNA, and miRNA. These molecules can target specific receptor cells and initiate signaling pathways that regulate various biological functions. In addition, EVs can transport large molecules between cells. Due to these properties, EVs offer unique opportunities for the development of new therapies. Notably, mesenchymal stem cell-derived EVs (MSC-EVs) are considered promising alternatives to stem cell transplantation therapy. MSC-EVs can stimulate angiogenesis, inhibit inflammation, resist apoptosis, and promote regeneration. This makes MSC-EVs potential candidates for the management of neurodegenerative diseases. The author’s research team recently completed the first clinical trial (NCT04388982) in patients with Alzheimer’s disease (AD), in which human adipose MSC-EVs were administered intranasally twice weekly for 12 weeks, followed by continuous monitoring to evaluate the safety and efficacy of MSC-EVs for AD treatment. Encouragingly, no adverse events were reported during visits in all groups. Additionally, Alzheimer’s Disease Assessment Scale (ADAS-cog) scores decreased by 2.33 ± 1.19 at week 12 compared to baseline, and Montreal Cognitive Assessment baseline scores increased by 2.38 ± 0.58, indicating improvements in cognitive function. Notably, the ADAS-cog score in the mid-dose group continued to decline until week 36 when it dropped by 3.98 points. This clinical trial highlights the potential of MSC-EVs in treating central neurodegenerative diseases. However, clinical translation of EV-based therapies still faces challenges such as low EV retention at the administration site and rapid systemic clearance. At the same time, limited production output and high costs also hinder the accessibility of EVs. Therefore, the key to future therapeutic applications must lie in the development of controllable, sustained delivery systems to prolong the retention time of EVs in the body and enhance their bioavailability.
Hydrogels have tunable delivery properties that can be precisely controlled by adjusting parameters such as material composition, degree of cross-linking and pore structure. This tunability enables hydrogels to achieve diverse release kinetics, from fast to slow release, directly affecting the efficacy and potential side effects of therapeutic agents. To improve the clinical translation potential of EVs, studies have designed hydrogel-based formulations. These formulations contain hydrophilic polymers that form cross-linked networks in aqueous solutions. However, conventional hydrogels typically release encapsulated drugs through the inherent but gradual disintegration of the hydrogel and diffusion of the drug. They lack the ability to modulate drug release, thus leading to reduced efficacy of EVs. To address the challenges faced by EVs delivery, there is an urgent need to develop a hydrogel system with controlled release properties, especially designed for EVs delivery.
Extracellular vesicles (EVs) are widely recognized as key mediators of local and long-distance intercellular communication. To achieve effective long-distance intercellular communication, EVs must exhibit excellent tissue/cell penetration capabilities, especially in the complex extracellular matrix (ECM). Permeability is largely facilitated by those EV membrane proteins with ECM-degrading activity. To overcome the challenges of EV release in hydrogels and enhance their functionality, the authors proposed the development of a smart hydrogel that can release EVs based on the enzymatic activity of EV membrane proteins. This hydrogel is expected to prolong the retention time of EVs at the administration site and achieve their controlled release. The self-stimulated release process not only enhances stability but also delays clearance and achieves a more reliable sustained effect, thereby improving EV bioavailability. These properties are crucial to expand application scenarios and enhance the clinical translation potential of EVs.
To test the above hypothesis, the author’s team used human adipose MSC-EVs as a model to construct a hydrogel responsive to MSC-EVs’ membrane proteins. They investigated the effect of hydrogels on the efficacy of MSC-EVs in treating Alzheimer’s disease (AD), which is the most common neurodegenerative disease and a major cause of dementia. To evaluate the potential of MSC-EVs’ membrane proteins as triggers for enzyme-catalyzed hydrogel degradation, the permeability of MSC-EVs was first verified. Compared to EVs derived from the microglial cell line BV2, MSC-EVs exhibited higher permeability. Subsequently, comparative proteomic analysis was conducted, identifying specific membrane proteins on MSC-EVs that exhibited matrix-degrading activity. Based on the enzymatic activity of MSC-EVs membrane proteins, the authors designed and synthesized a hydrogel composed of self-assembling peptides that is easily degraded by membrane enzymes present in MSC-EVs. After intranasal administration, this smart release hydrogel extended the retention time of MSC-EVs at the administration site, released MSC-EVs in a controlled manner, and significantly enhanced the efficacy and clinical translational potential of MSC-EVs for AD treatment. This study proposes a smart hydrogel design method based on comparative proteomics that can significantly improve the applicability of EVs in clinical settings.
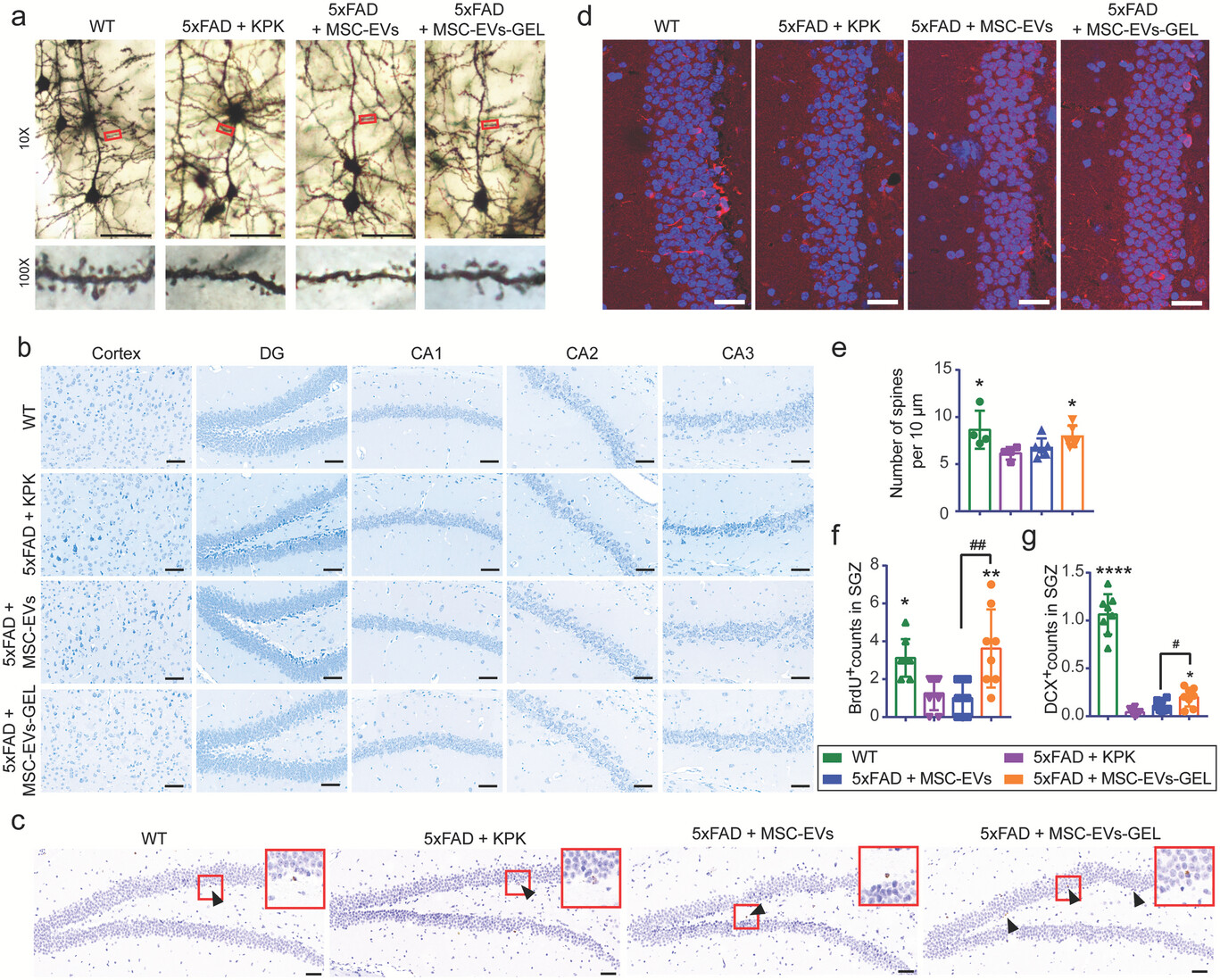
MSC-EVs-GEL more effectively alleviate neuronal damage and promote neurogenesis in 5×FAD AD model mice.
Reference:
Huang M, Zheng M, Song Q, et al. Comparative Proteomics Inspired Self-Stimulated Release Hydrogel Reinforces the Therapeutic Effects of MSC-EVs on Alzheimer’s Disease. Adv Mater. Published online December 29, 2023. doi:10.1002/adma.202311420
Related Services:
