Extracellular vesicles (EVs) secreted by all cell types are involved in the intercellular communication, delivering regulatory factors that influence cell and tissue phenotypes in both normal and diseased tissues. Therefore, EVs represent a promising source of biomarker targets for disease diagnosis in blood and samples. However, the sensitive detection of biomarkers from EVs originating from specific cell populations remains a significant challenge in the analysis of complex biological samples. Researchers from Tulane University School of Medicine have compiled current innovative methods to simplify the isolation of EVs and discuss how to improve the sensitivity of EV detection procedures needed for clinical applications in EV-based genetic diagnosis and therapy. These methods encompass cutting-edge technologies such as nanotechnology and microfluidic techniques, facilitating the characterization of EVs. Furthermore, the study outlines both the opportunities and challenges in translating of EV genetic testing into clinical practice. This relevant study was published online on March 12 in ACS Nano, a prestigious academic journal renowned for its contributions to the field of nanomaterials, under the title “A Panorama of Extracellular Vesicle Applications: From Biomarker Detection to Therapeutics”.
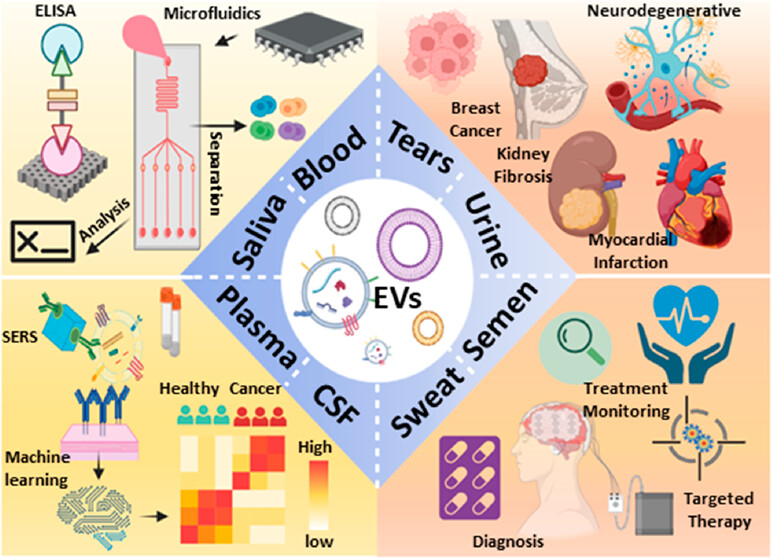
Extracellular vesicles (EVs) play an important role in intercellular communication and regulation, influencing processes related to disease pathology. Given their widespread secretion by various cell types, EVs are implicated in both local and systemic cytopathological responses. Rapid, accurate, and sensitive detection methods are developed by targeting EV-specific biomarkers to detect specific EV populations associated with disease processes. EVs, abundant in most body fluids, simplify diagnostic sample collection, carrying a plethora of biomolecules such as proteins, mRNA, miRNA, lncRNA, DNA, lipids, and metabolites. These biomarkers, derived from EVs secreted by damaged or diseased cells, harbor rich diagnostic and prognostic information, governing intercellular communication, maintenance, cell maturation, and immune responses related to disease development and pathology. Therefore, EV-derived biomarkers are proposed candidates for infectious, chronic, and malignant diseases.
However, sensitive and accurate detection of specific EV biomarkers is complicated by the presence of diverse EV populations and nanoparticle contaminants (protein aggregates, lipoproteins, cell debris, etc.) in biological samples. Single EV analysis methods aim to circumvent this issue, enabling evaluation of the complete spectrum of EVs and their potential roles in disease pathology. However, this approach is still nascent due to incomplete understanding of EV biogenesis, technical limitations, and a lack of standardization and validation methods. Extensive research is directed towards developing EV-based therapeutics capitalizing on their desirable properties, including stability, tissue permeability, cell tissue selectivity, and drug loading capacity, to modulate disease development and progression. This involves modifying the content or surface molecules of specific EV populations through genetic engineering, transfection/fusion, or chemical modification to alter their regulatory functions or cellular targeting specificity. However, challenges persist in clinical EV diagnostic applications, encompassing difficulties in sample collection, purification, quantification, processing, isolating EVs for specific cell types, and understanding their pathological effects. Guidelines such as the “Minimum Information for Research on Extracellular Vesicles” (MISEV) offer recommended procedures for EV characterization, processing, and analysis, streamlining the development of clinically-ready EV therapeutics. Similarly, method-specific guidance aids in driving the development of diagnostic tools using standardized techniques like quantitative real-time polymerase chain reaction (qPCR) and flow cytometry, and other specialized methods that require standardized guidance to produce reliable data. Leveraging automated systems for EV isolation and characterization also accelerates the development of clinically feasible EV-based diagnostic applications.
Further development of standardized EV handling procedures is still necessary to ensure the preservation of biomarker integrity before analysis, especially in early diagnostic samples where the signal intensity might be very low due to the assessment of low concentrations of EVs associated with disease development or progression. This is especially true in longitudinal samples of biomarkers. Additionally, standardized EV preparation and characterization methods are imperative to ensure the consistent safety and efficacy of EV treatments. Moreover, additional studies are warranted to pinpoint EV biomarkers associated with effective treatment response, although “omics” studies can provide important insights into this issue. Clinical applications using EVs for diagnosis or therapy demand rational approaches that simplify workflows and improve the performance of current methods. Consequently, researchers undertook a comprehensive review of this field, describing the advancements and persistent challenges encountered in EV-based diagnosis and treatment. In this review, researchers mainly discussed in detail the following aspects:
1. Advancements in EV Detection Technology
Traditional EV analysis methods, including polymerase chain reaction (PCR), Western blotting (WB) and enzyme-linked immunosorbent assay (ELISA), are known for their time-consuming and labor-intensive, have moderate sensitivity, and requirement for separate EV purification steps, thereby limiting their ability for clinical applications. However, tremendous progress has been made in recent years to improve EV analysis methods, aiming to augment their sensitivity, specificity and utility in clinical settings. Customized nanomaterials and microfluidic platforms are currently under investigation to further enhance their performance characteristics . EV detection methods such as fluorescence, surface plasmon resonance (SPR), surface-enhanced Raman spectroscopy (SERS), electrochemistry and aptamers can all be combined to achieve optimal performance on the EV detection platform, facilitating sensitive signal recognition.
2. Single EV Analysis Technology and Its Diagnostic Potential
In recent years, there has been a concentrated effort in translational EV research towards the development of advanced methods capable of biophysical characterization at the single EV level. EVs present in biological samples exhibit significant heterogeneity, encompassing phenotypes that mirror diverse cell types across various tissues. For example, plasma EVs may originate from cells representing different lineages or fate states within a tissue, or even cells with distinct genetic backgrounds within a tumor. Techniques focusing on individual EV profiling can elucidate aspects of this heterogeneity among EV subpopulations, such as size and composition. They are adept at detecting low concentrations of EV biomarkers in complex samples and establishing associations with specific EV biomarkers on individual EVs or EV subpopulations. Such analyses furnish invaluable insights into disease development and progression or treatment response, thus enriching the diagnostic potential of EV analysis in complex samples. Single EV analysis methods encompass a diverse array of techniques, several of which do not require the use of exogenous labels. These include nanoparticle tracking analysis (NTA), cryo-electron microscopy (cryo-EM), atomic force microscopy (AFM), and laser tweezer Raman spectroscopy. However, many of these techniques offer limited label-free information or may falter in detection without independent EV isolation procedures. For instance, label-free methods like NTA only provide an estimation of the EV diameter distribution within the population. While cryo-electron microscopy and atomic force microscopy can provide some insights into the conformational phenotypic distribution, all three methods might struggle to distinguish EVs from other entities like protein aggregates and viruses that could contaminate EV isolates. Consequently, an increasing number of individual EV analysis methods are incorporating fluorescent affinity tags to identify specific EV subpopulations for analysis.
3. Diagnosis, Prognosis, and Therapeutic Applications of EVs
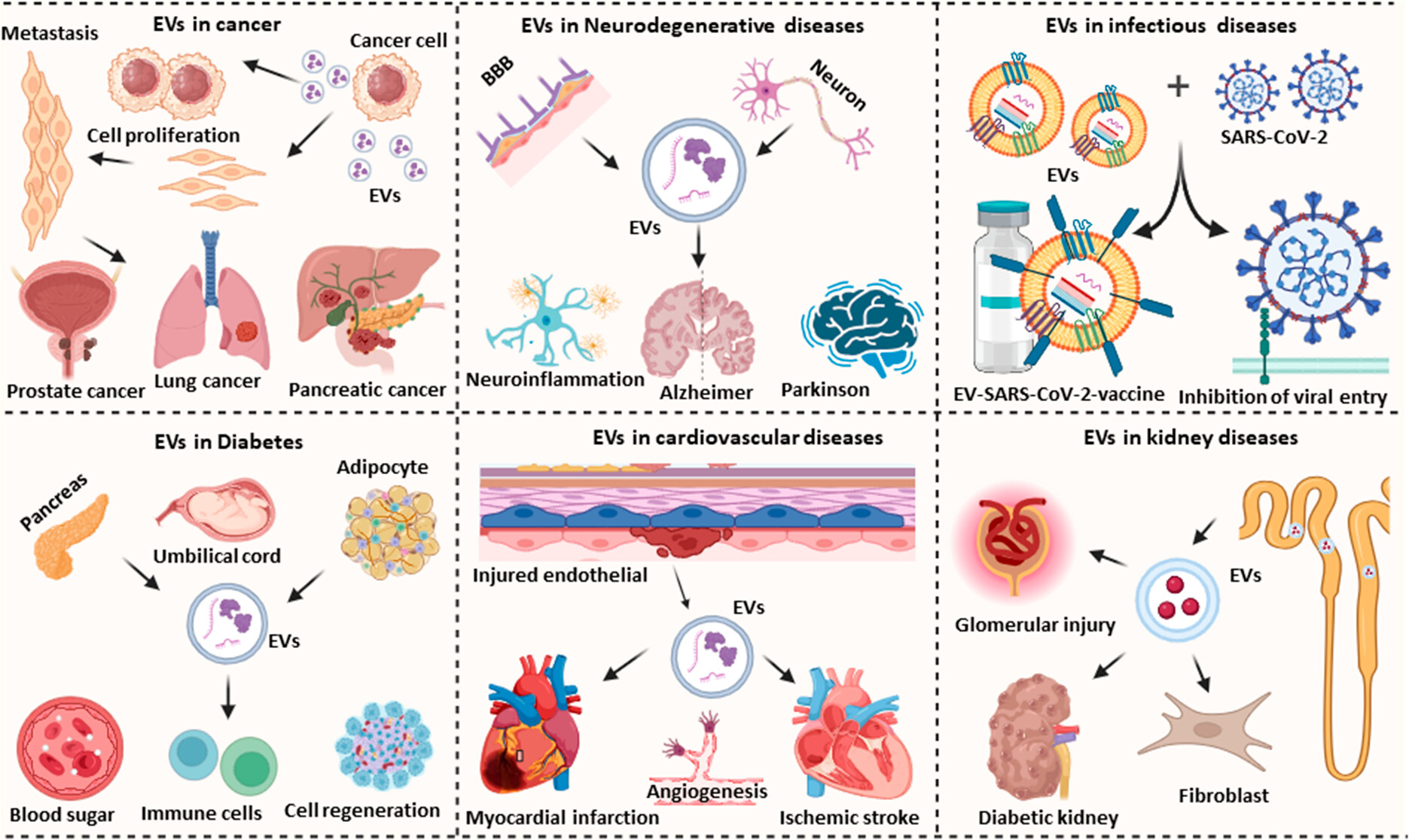
EVs have great potential in diagnostic applications across infectious, chronic and malignant diseases due to their ability to discern and differentiate specific diseases as well as discrete disease stages. Additionally, they reflect cellular responses to therapeutic interventions through changes in disease-associated EV biomarkers. Enhanced EV isolation and characterization methodologies are poised to refine EV diagnostics and therapeutics in development, thereby augmenting their prospects for clinical translation. Notably, machine learning methods show promise in discerning and characterizing specific EV populations.
Reference:
Das S, Lyon CJ, Hu T. A Panorama of Extracellular Vesicle Applications: From Biomarker Detection to Therapeutics. ACS Nano. 2024 Mar 12. doi: 10.1021/acsnano.4c00666. Epub ahead of print. PMID: 38471757.
Related Services:
