Extracellular vesicles (EVs) are biological nanoparticles that mediate short- and long-distance intercellular communication, delivering bioactive payloads. Short-distance communication occurs in the interstitium, while long-distance communication necessitates transport through the blood circulation to reach distant sites. Extracellular vesicle therapies are commonly administered via systemic injection, and diagnostic methods often rely on detecting of organ-derived EVs in the blood. However, the mechanisms by which EVs enter and exit the circulation are still unclear. Recently, a review was published in the journal Nature Nanotechnology (IF=38.3), discussing the lymphatic system and transport across the endothelial barrier through intracellular and intercellular pathways as potential pathways for EVs to enter and exit the blood circulation system.

Although many studies have reported the presence of organ-specific EVs in the blood, the mechanisms by which these EVs exit the interstitium remain mostly unknown. Similarly, while systemically administered EVs have been shown to reach specific organs, evidence for crossing the endothelial barrier remains unclear. The exponential growth of the EV field and the accelerated development of emerging therapeutic and diagnostic EV-based products highlight the urgent need to understand EV transport within the body. Therefore, the phenomenon of EV transport can be considered the most critical component of the desired functionality, as the wrong spatial environment is futile and may be harmful. This review explores the potential nanoscale processes by which EVs enter and exit the blood circulation.
Most organs’ capillaries have a nonfenestrated continuous endothelial lining that only allows water, small-molecule solutes, and gases to pass through. In contrast, organs such as the liver and spleen have discontinuous endothelial linings that allow macromolecules and nanoscale particles to transit between the interstitial space and the blood circulation. The presence of various subtypes of endothelium in the same organ is common, for instance, the kidney, which contains several endothelial cell types with different structures and roles. Renal endothelial cells found in medium and large blood vessels form nonfenestrated continuous layers interconnected by intercellular junctions and extending in the direction of blood flow. In contrast, glomerular endothelial cells have numerous fenestrations and a thick glycosylated layer, contributing to the filtration properties of the glomerular filtration barrier. Endothelial cells of proximal tubule capillaries have fenestrations and are covered by a membrane made of glycoproteins, facilitating the reabsorption and secretion of fluids and substances from adjacent tubule epithelial cells. Endothelial cell heterogeneity within and between organs may significantly impact the mechanisms by which EVs cross the endothelial barrier, and this review discusses possible routes.
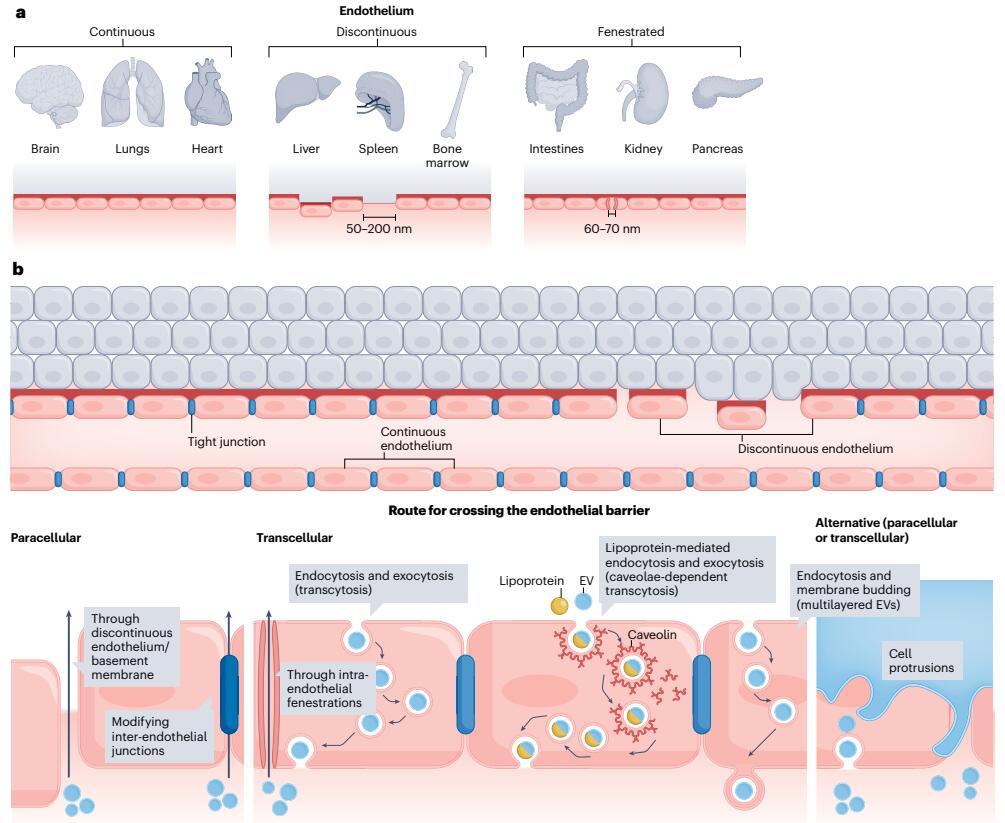 Fig. 1 Potential Pathways of EV Entry into and Exit from the Blood Circulation a.General representation of endothelium types. It’s worth noting that several types of endothelia can be found in some organs, such as the kidneys. b.Extracellular vesicles may cross the endothelial barrier through transcellular or paracellular transport routes. In the case of paracellular transport, EVs may pass through gaps between endothelial cells, likely a prominent transport route in organs with discontinuous vasculature, such as the liver. EVs may also modify interendothelial junctions to enable paracellular transport. Concerning transcellular transport, EVs may pass through intra-endothelial fenestrations or use various transcytosis pathways. Exploiting binding to lipoproteins for hijacking caveolae-mediated transcytosis may also be occur. Exocytosis-independent mechanisms of transcellular transport are plausible, resulting in multilayered EVs released through membrane budding. Finally, cell protrusions can potentially be exploited as an alternative route for EV transport across the endothelial barrier.
Fig. 1 Potential Pathways of EV Entry into and Exit from the Blood Circulation a.General representation of endothelium types. It’s worth noting that several types of endothelia can be found in some organs, such as the kidneys. b.Extracellular vesicles may cross the endothelial barrier through transcellular or paracellular transport routes. In the case of paracellular transport, EVs may pass through gaps between endothelial cells, likely a prominent transport route in organs with discontinuous vasculature, such as the liver. EVs may also modify interendothelial junctions to enable paracellular transport. Concerning transcellular transport, EVs may pass through intra-endothelial fenestrations or use various transcytosis pathways. Exploiting binding to lipoproteins for hijacking caveolae-mediated transcytosis may also be occur. Exocytosis-independent mechanisms of transcellular transport are plausible, resulting in multilayered EVs released through membrane budding. Finally, cell protrusions can potentially be exploited as an alternative route for EV transport across the endothelial barrier.
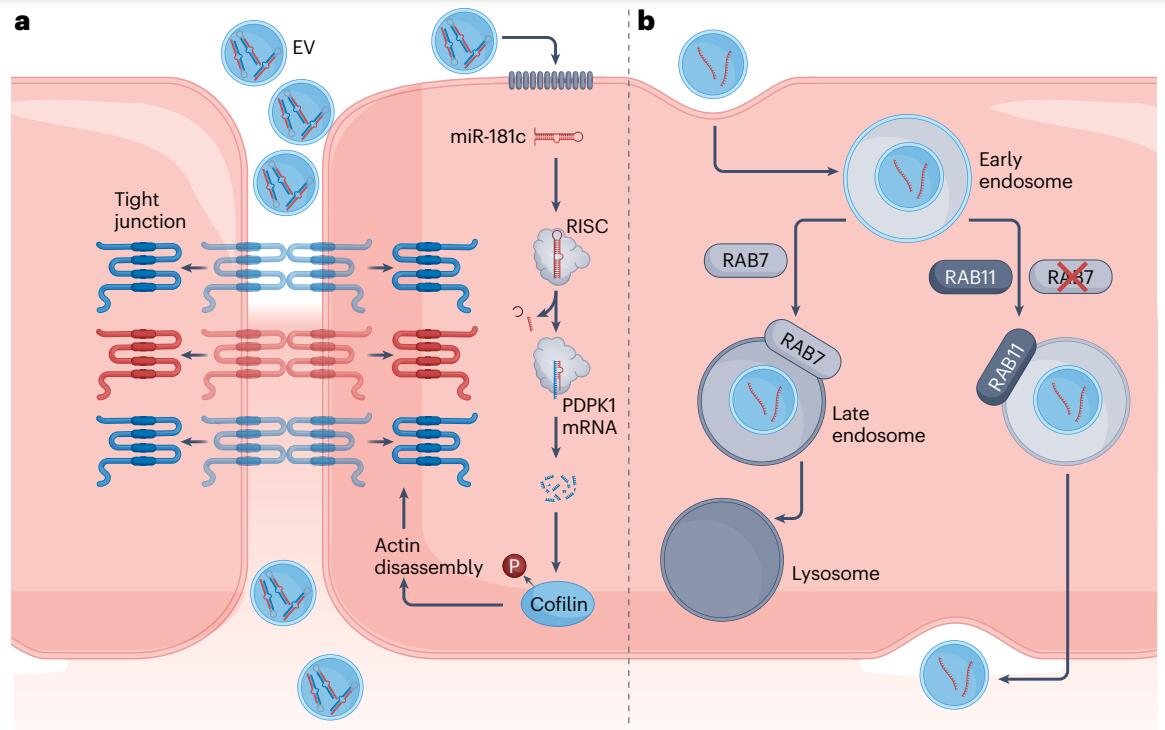 Fig. 2 Mechanisms for Cancer Cell-Derived EVs to Cross the Blood-Brain Barrier Endothelium a. Cancer EV-associated miR-181c modulates the integrity of the tight junction by downregulating the expression of PDPK1, a protein involved in the phosphorylation of cofilin17. Increased cofilin dephosphorylation, as a result of this process, leads to eventual actin filament disassembly, displacing attached tight junction proteins from their transmembrane localities into the cytoplasm. This causes increased vascular permeability for the paracellular movement of EVs. b. Breast cancer-derived EVs have been found to suppress the RAB7-associated degradation pathway following endocytosis, redirecting themselves to the RAB11-associated recycling pathway36. This enables subsequent transcytosis of the EVs through the basolateral membrane of the endothelial cells, into the interstitium. mRNA; messenger RNA; RISC, RNA-induced silencing complex; P, phosphate group; RAB, Ras-associated binding (protein).
Fig. 2 Mechanisms for Cancer Cell-Derived EVs to Cross the Blood-Brain Barrier Endothelium a. Cancer EV-associated miR-181c modulates the integrity of the tight junction by downregulating the expression of PDPK1, a protein involved in the phosphorylation of cofilin17. Increased cofilin dephosphorylation, as a result of this process, leads to eventual actin filament disassembly, displacing attached tight junction proteins from their transmembrane localities into the cytoplasm. This causes increased vascular permeability for the paracellular movement of EVs. b. Breast cancer-derived EVs have been found to suppress the RAB7-associated degradation pathway following endocytosis, redirecting themselves to the RAB11-associated recycling pathway36. This enables subsequent transcytosis of the EVs through the basolateral membrane of the endothelial cells, into the interstitium. mRNA; messenger RNA; RISC, RNA-induced silencing complex; P, phosphate group; RAB, Ras-associated binding (protein).
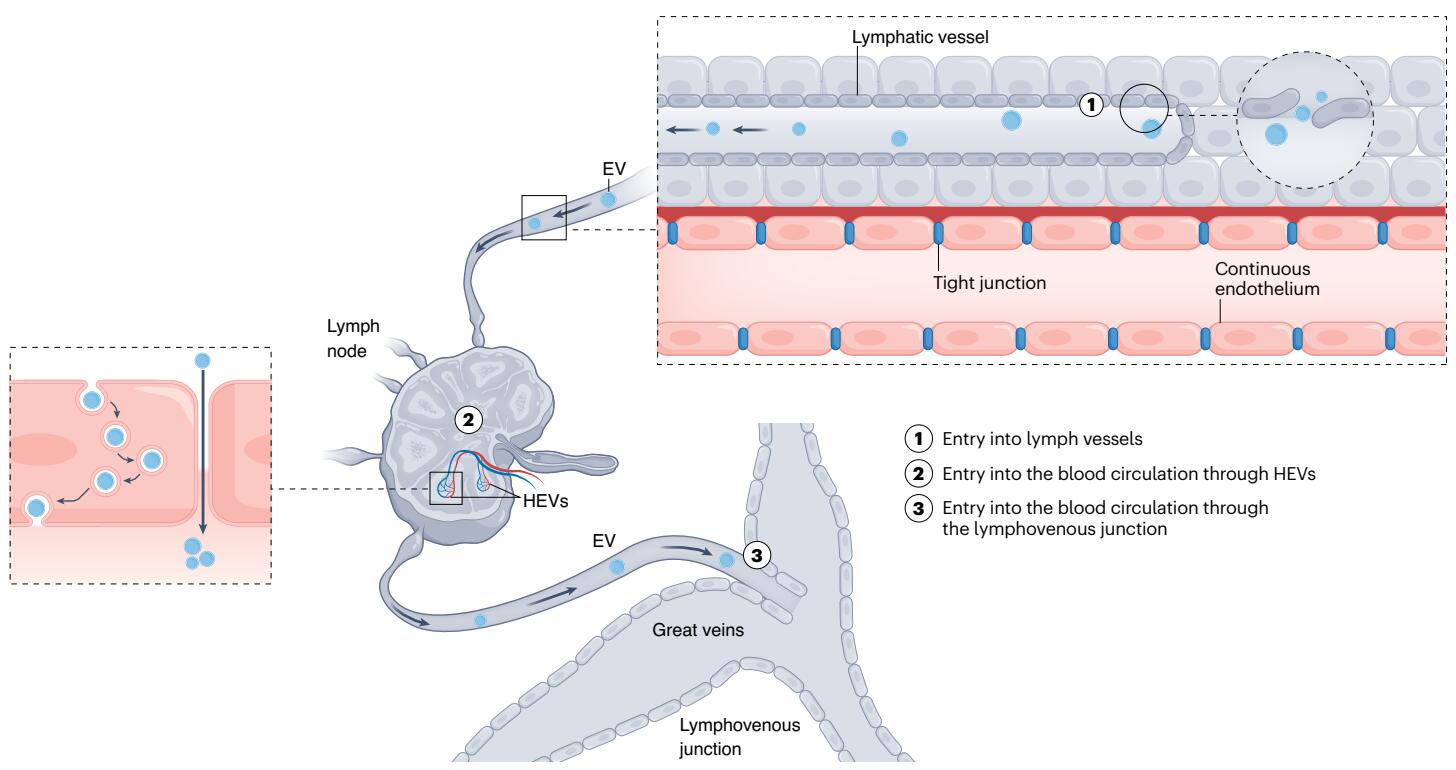 Fig. 3 Potential Pathways of EV Entry into the Blood Circulation Through the Lymphatic System Extracellular vesicles may exit the interstitium through fluid-pressure-sensitive junctions (flap valves) in the lymphatic endothelium that create micrometer-sized gaps (1), subsequently entering the blood circulation either by exiting through high endothelial venules (HEVs) in the lymph nodes (2) or through the lymphovenous junction that drains into the great veins (3).
Fig. 3 Potential Pathways of EV Entry into the Blood Circulation Through the Lymphatic System Extracellular vesicles may exit the interstitium through fluid-pressure-sensitive junctions (flap valves) in the lymphatic endothelium that create micrometer-sized gaps (1), subsequently entering the blood circulation either by exiting through high endothelial venules (HEVs) in the lymph nodes (2) or through the lymphovenous junction that drains into the great veins (3).
Taken together, numerous possible mechanisms exist by which EVs communicate among the interstitium, lymphatic system, and blood circulation. Although individual examples of interstitial and intracellular transport have been described, a comprehensive understanding of EVs entering and exiting the blood circulation under physiological and pathological conditions is still lacking. This review underscores the urgent need to study EV transport across vascular barriers, critical for understanding intercellular communication beyond the local interstitium and exploiting EV biology for diagnostic and therapeutic uses.
On the therapeutic side, understanding EV transport can help select the most effective route of administration for a given application. Subcutaneous, peritoneal, and intramuscular routes may enhance lymphatic transport, as demonstrated with synthetic nanoparticles. However, insufficient understanding of the fate of EVs after lymphatic drainage has limited the development of delivery methods to improve transport and therapeutic efficacy. Furthermore, the impact of EV physical properties (such as size) on their interaction with the vascular barrier remains mostly unknown, hampering the rational design of ideal treatment options. In animal models, small EVs (approximately <100 nm) and large EVs (approximately >200 nm) exhibit different distribution and accumulation characteristics upon administration. For example, a systematic review of biodistribution studies showed that elevated deposition of small EVs was observed in the liver and kidneys within the first hour after administration, and in the lungs and spleen within 2–12 hours. Large EVs, on the other hand, showed increased accumulation in the lungs (first hour) and liver (2-12 hours). However, the EV transport phenomena and vascular interactions that mediate different biodistribution profiles remain unclear. Understanding EV transport is also crucial for diagnostic applications. For example, based on the importance of lymphatic transport, diagnostic capabilities could be improved by sampling EVs from lymph nodes and screening for disease-related proteins, nucleic acids, and lipids. In conclusion, lymphatics provide a promising avenue for diagnostic and therapeutic purposes as they are likely to be the main route for EV communication.
Reference:
Iannotta D, A A, Kijas AW, Rowan AE, Wolfram J. Entry and exit of extracellular vesicles to and from the blood circulation. Nat Nanotechnol. 2023 Dec 18. doi: 10.1038/s41565-023-01522-z. PMID: 38110531.
Related Services:
Body Fluid Exosome & Tissue Exosome Combined Research Service
Exosome Cargo Loading Services
