In 50 families from the Netherlands, the United Kingdom, the United States, and China, each family has a child paralyzed due to a mutation in the Cntnap1 (Contactin-Associated Protein 1) gene. These children are immobile, requiring assistance with feeding, diaper changes, and constant monitoring by caregivers. Human CNTNAP1 mutations are associated with hypomyelinating neuropathy-3, a condition leading to severe neurological dysfunction related to myelin sheath development.
In a recent study, Dr. Manzoor Bhat and his team at the University of Texas Health Science Center at San Antonio made a discovery that offers hope for these severely affected children through gene therapy. The research findings were published online on October 19, 2023, in the journal Cell Reports under the title “Mouse models of human CNTNAP1-associated congenital hypomyelinating neuropathy and genetic restoration of murine neurological deficits.”
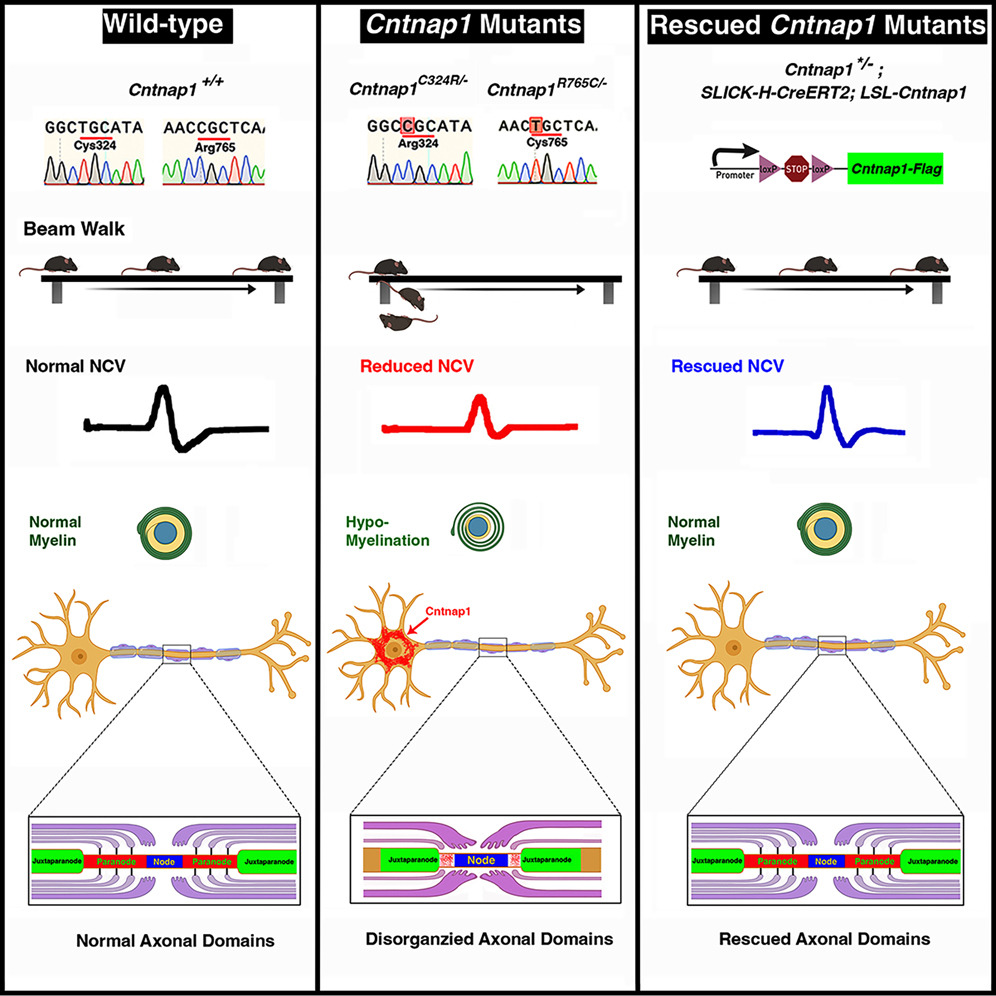
Bhat stated, “We obtained genetic information from these families and created transgenic mice models that could replicate the human mutations and diseases. Our mice exhibited phenotypes or weaknesses similar to these children.” Bhat is a leading figure in the field of neuron-glial biology, and his laboratory discovered the mouse Cntnap1 gene in 2001.
Transgenic animals refer to animals in which foreign genes are introduced into the genome. The transgenic mice developed by Bhat’s lab carry both normal copies of the Cntnap1 gene and mutant copies reflecting the mutations observed in these children. Bhat explained, “We can control when the normal gene is turned on. It turns out we can use the normal gene to rescue the neural defects in these mice.”
In a series of experiments, the researchers activated the normal Cntnap1 gene in mice at birth, five days after birth, two weeks after birth, one month after birth, and three months after birth. The earlier the normal Cntnap1 gene was activated, the quicker the mice’s condition improved, and the rescue was more thorough.
Bhat noted, “The longer we waited, the worse the condition of these mice. This is because the Cntnap1 gene produces a protein that drives neural impulse conduction. If we wait for a month or two, neural function becomes weak, muscles become weak, and the mice can’t maintain motor coordination.”
The mice were placed on beams to measure their movement. Over time, they navigated the beam more easily after the activation of the normal Cntnap1 gene, as the protein produced by the normal gene improved neural signal conduction.
The next phase of this new research involves injecting a virus into Cntnap1 mutant mice that can produce the Cntnap1 protein. If preclinical studies show promising results, the next step will be gene therapy for children.
Reference
1. Chang, Cheng, et al. “Mouse models of human CNTNAP1-associated congenital hypomyelinating neuropathy and genetic restoration of murine neurological deficits.” Cell Reports 42.10 (2023).
