Gene therapy is a revolutionary approach for treating severe genetic diseases, typically involving the replacement or repair of faulty genes. Currently, the most effective delivery system is based on adeno-associated virus vector, which naturally possesses the ability to introduce genetic information into human cells.
One of the biggest challenges scientists face in transitioning gene therapy from the lab to the clinic is the lack of effective preclinical models available for developing and testing new therapies, such as laboratory tests with biological relevance and clinical predictability conducted before applying a therapy to patients. These models must faithfully replicate human physiological conditions and complex tissue structures to accurately predict the outcomes of treatment in patients.
Previously, scientists established a novel method for maintaining the viability of human livers in a laboratory environment. Livers unsuitable for transplantation—previously discarded or stored on ice for research—are now able to be preserved ex vivo at body temperature, enabling cutting-edge biomedical research. This is known as the normothermic liver perfusion system.
In a groundbreaking global study, researchers from institutions including the University of Sydney in Australia tested a novel gene therapy within intact human livers, aiming to develop more effective methods for treating life-threatening genetic diseases. The related research findings were published in the journal Nature Communications on March 14, 2024, titled “Harnessing whole human liver ex situ normothermic perfusion for preclinical AAV vector evaluation.”
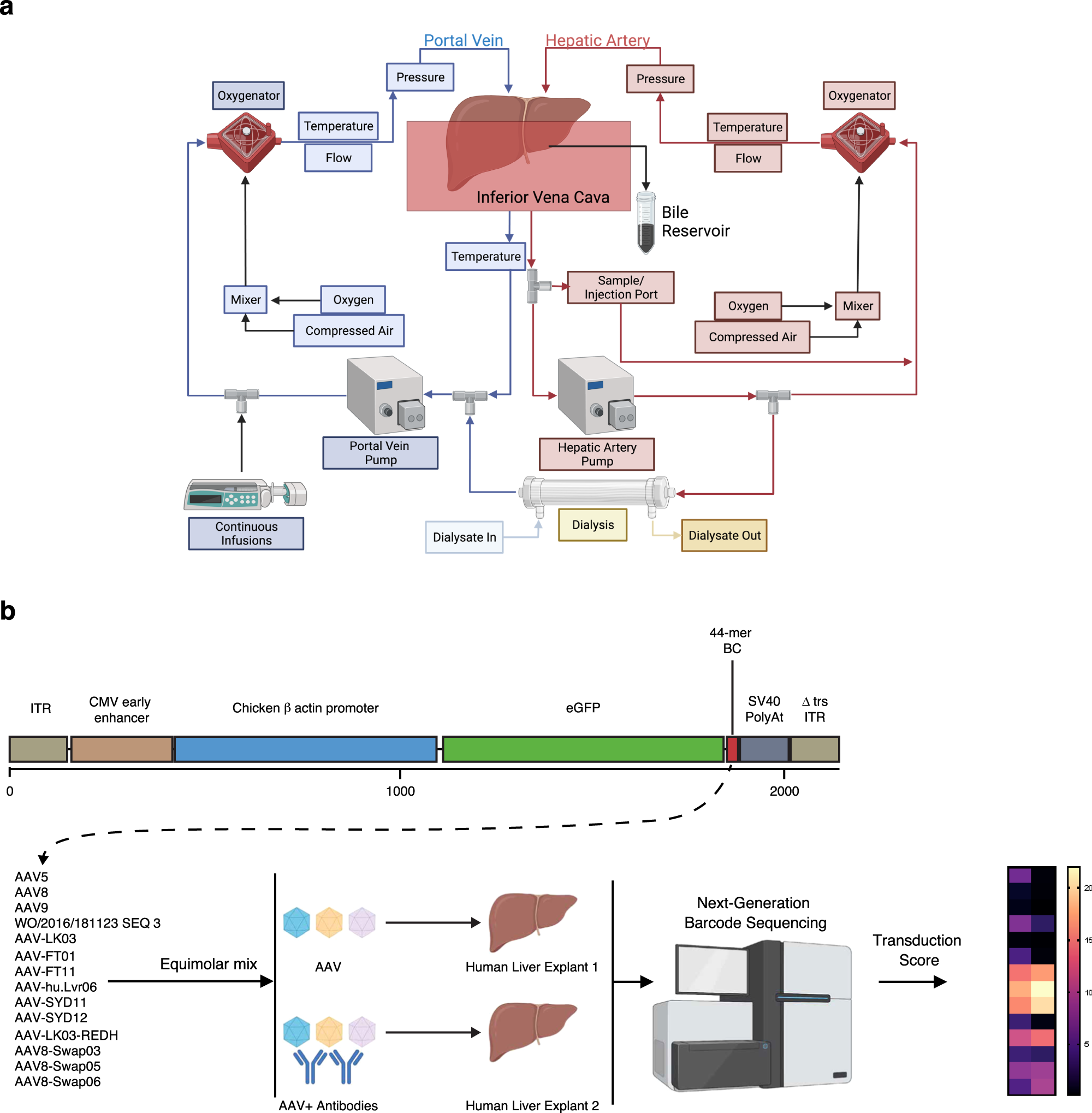
These authors confirmed that testing AAV-based therapies using this system before initiating clinical studies is feasible. Utilizing intact human livers represents a revolutionary advancement in the field of gene therapy because it allows for precise testing of how new therapies will impact a major organ—the liver—in a manner previously unattainable.
Associate Professor Leszek Lisowski, the corresponding author of the paper, said, “This is tremendously exciting because for the first time we can directly evaluate the function of gene therapy in a clinical target organ—the human liver.”
He added, “This is critical because the viral vectors we currently use for delivering gene therapy to the liver are not yet sufficient for most clinical applications. Currently, we often have to deliver high doses to overcome their low functionality and achieve clinical efficacy. Gene therapy delivery tools have been tested in animal models thus far, and while animal models are valuable for assessing safety and targeting other organs/tissues, they do not fully recapitulate the functionality of these delivery methods in patients.”
He further stated, “This research expands the scope of preclinical models available for liver-targeted vector research, allowing us to minimize the use of animals. Ideally, this will bring us closer to providing more effective gene therapy for diseases that currently have limited treatment options.”
Lisowski noted that this new advanced human liver model not only changes the game for functional assessment of novel therapies but also allows for more accurate estimation of effective doses of new therapies and identification of potential toxic side effects. It will also serve as a robust system for developing novel viral vectors, laying the foundation for the next generation of advanced therapies.
What We Do
Creative Biolabs provides comprehensive viral vectors and cutting-edge viral vector technology for basic research and preclinical applications, including the design and construction of suitable viral vectors and small to large-scale production of viral vectors. Our custom viral vector production stands at the forefront of gene delivery system discovery, offering a varied range of vectors that are optimized to align your project specifics while ensuring high transduction efficiency, specificity, and safety.
• Lentivirus Vector
• Adenovirus Vector
• Adeno-Associated Virus Vector
• Herpes Simplex Virus Vector
• Vaccinia Viral Vector
• Baculovirus Vector
• Alphavirus Vector
• Flavivirus Vector
• Measles Virus Vector
• Foamy Virus Vector
• Hybrid Adenoviral Vector
• Helper-Dependent Adenoviral Vector
Reference
1. Cabanes-Creus, Marti, et al. “Harnessing whole human liver ex situ normothermic perfusion for preclinical AAV vector evaluation.” Nature Communications 15.1 (2024): 1876.
