Recently, researchers from the Utrecht University Medical Center published a research paper in the Nature subsidiary journal, Nature Cardiovascular Research, titled: “Therapeutic efficacy of AAV-mediated restoration of PKP2 in arrhythmogenic cardiomyopathy.” The study, which utilizes recombinant adeno-associated virus delivery of the PKP2 gene for treatment, lays the foundation for gene therapy in genetic heart diseases—specifically, arrhythmogenic cardiomyopathy (ACM). This therapy is scheduled for multiple clinical trials in the United States in 2024.
Arrhythmogenic cardiomyopathy (ACM) is often caused by mutations in genes associated with desmosomes, which are responsible for connecting adjacent cardiac muscle cells. Not only do they provide structural connectivity, but they also ensure the synchronized contraction of cardiac muscle cells, allowing the heart to pump blood in a coordinated manner.
The most common mutation in ACM affects the PKP2 gene, which encodes for an essential component of the desmosome—plakophilin-2. Patients with this mutation typically have lower levels of plakophilin-2 protein in cardiac muscle cells, leading to weakened intercellular connections, making it difficult for them to work in sync, and resulting in the development of arrhythmias.
Therefore, the research team considered developing a gene therapy targeting the PKP2 gene mutation to fundamentally treat arrhythmogenic cardiomyopathy (ACM). Introducing the correct PKP2 gene into affected cardiac muscle cells could potentially restore plakophilin-2 protein levels to normal, thereby strengthening the desmosomes and reducing the incidence of arrhythmias in these patients.
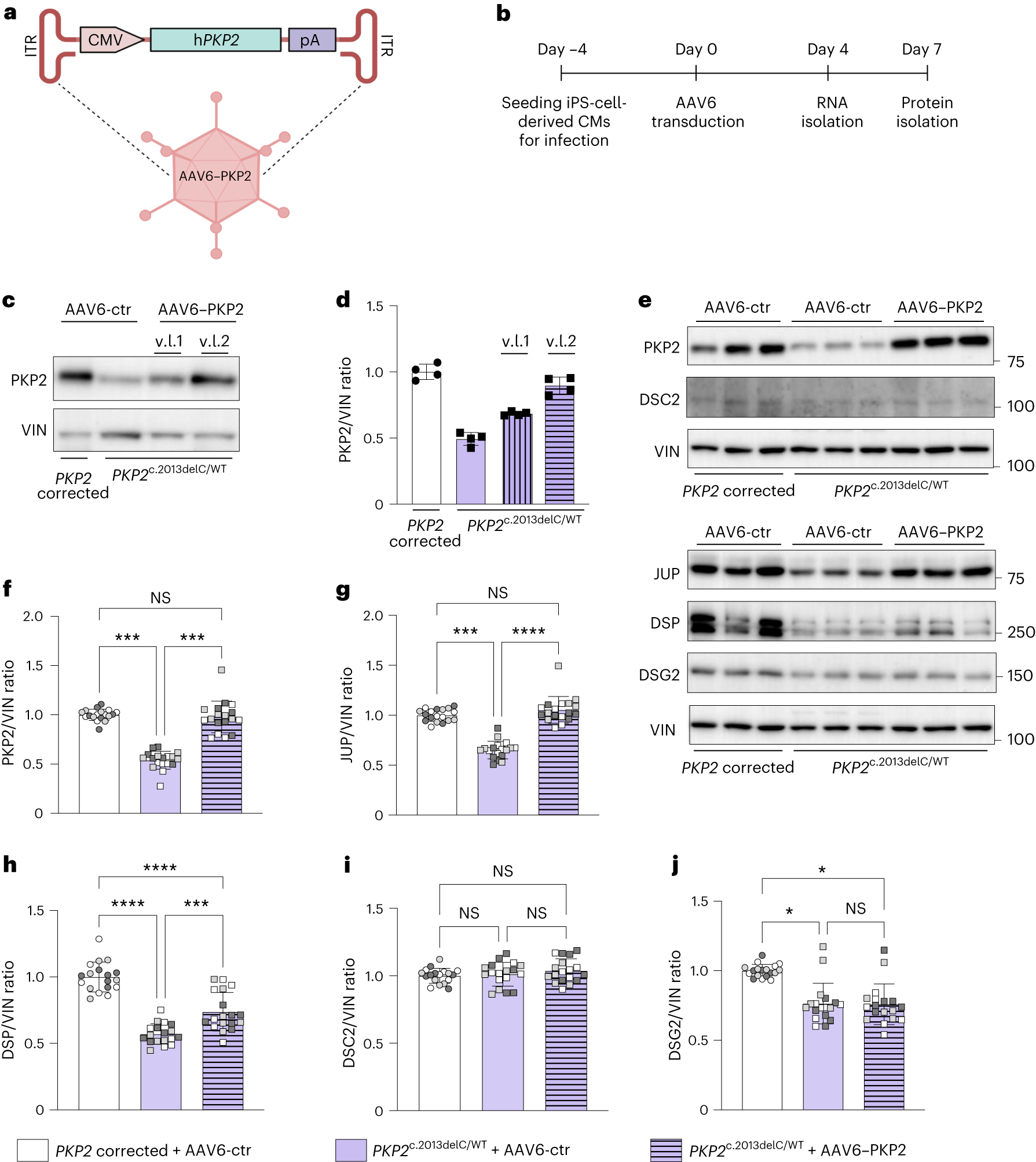
Using several laboratory models of arrhythmogenic cardiomyopathy (ACM), the research team demonstrated that delivering the correct PKP2 gene to human cardiac muscle cells derived from stem cells restored plakophilin-2 levels and improved their sodium ion conductance, which is important for the contractile ability of cardiac muscle cells.
Next, the research team confirmed the therapy’s beneficial effects on cardiac muscle contractility in laboratory-cultured engineered human cardiac muscle cells. Finally, the effectiveness of PKP2 gene therapy was further validated in a mouse model of arrhythmogenic cardiomyopathy (ACM), successfully improving the desmosomes and cardiac function recovery in the mouse model.
Following promising preclinical research progress, the next step is to explore the clinical therapeutic potential of this gene therapy method in patients with arrhythmogenic cardiomyopathy (ACM) carrying PKP2 gene mutations.
Eirini Kyriakopoulou, the lead author of the paper, stated that three companies in the United States have announced that they will start clinical trials next year to test the therapeutic effects of this gene therapy in patients. Once arrhythmogenic cardiomyopathy (ACM) progresses to a certain extent, part of the myocardium has already been replaced by fatty tissue, and whether this method can reverse existing myocardial damage remains uncertain. However, it may be sufficient to prevent early disease progression to more severe stages.
Although preclinical experimental results and the upcoming clinical trials bring great hope for arrhythmogenic cardiomyopathy (ACM), the true commercialization of this therapy may still take several years. In addition to confirming its efficacy in human patients, any safety concerns must be addressed and resolved before clinical application.
Reference
1. Kyriakopoulou, Eirini, et al. “Therapeutic efficacy of AAV-mediated restoration of PKP2 in arrhythmogenic cardiomyopathy.” Nature Cardiovascular Research (2023): 1-15.
