In a new study, researchers from University College London have developed a novel gene therapy to treat a devastating form of childhood epilepsy, showing significant reduction in seizure episodes in mouse models. This discovery holds promise for providing an alternative to surgery for children suffering from focal cortical dysplasia. The findings were published in the journal Brain, with the paper titled “Anti-seizure Gene Therapy for Focal Cortical Dysplasia.”
Focal cortical dysplasia is caused by regions of abnormal brain development and is one of the most common reasons behind drug-resistant epilepsy in children. It often occurs in the frontal lobes, which are crucial for planning and decision-making. Epilepsy associated with focal cortical dysplasia is linked to comorbidities, including learning disabilities. Although surgical removal of the affected brain malformation is effective, its use is severely limited by the risk of permanent neurological deficits, and it does not always prevent the recurrence of epilepsy.
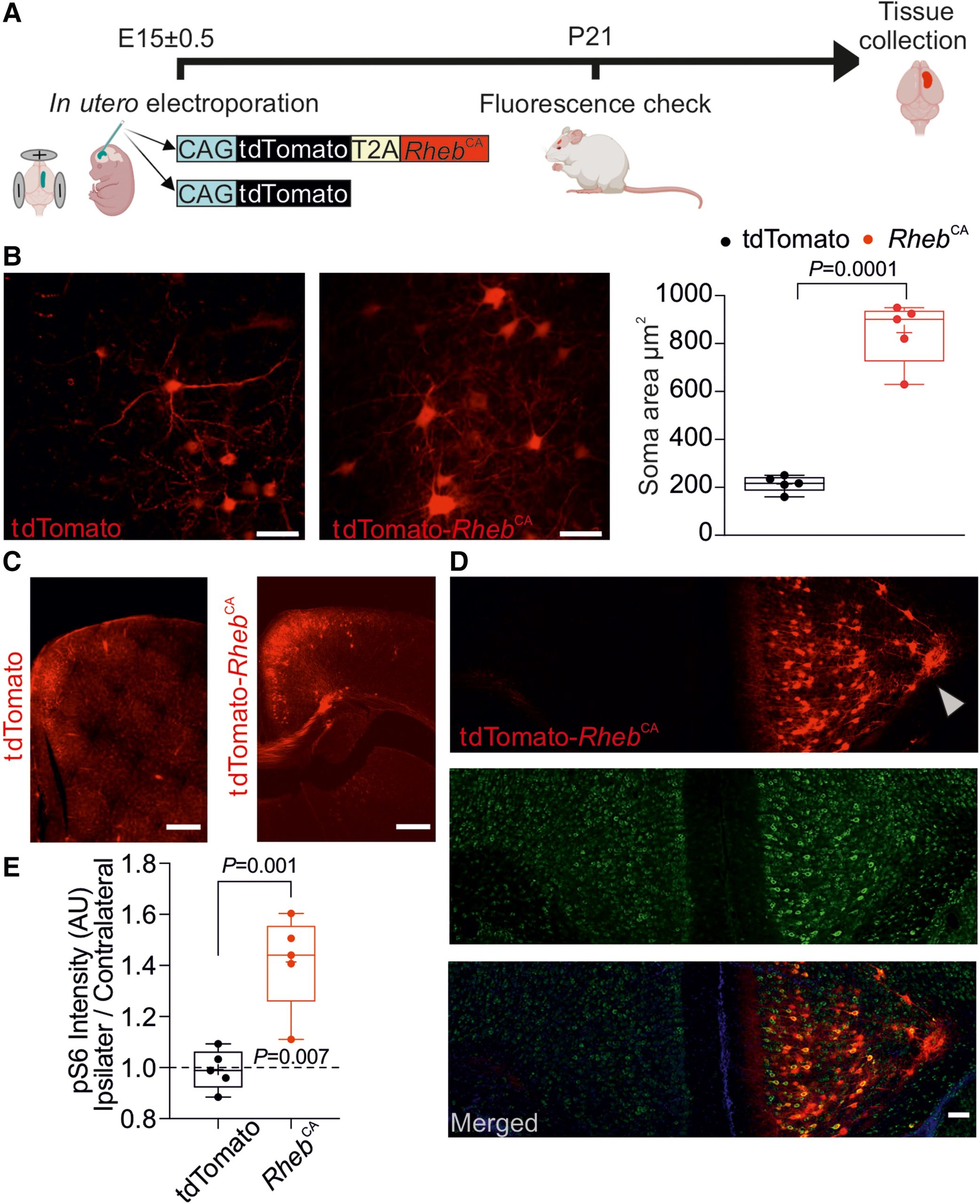
Therefore, in this new study, the authors evaluated a gene therapy in mouse models of frontal lobe focal cortical dysplasia. This therapy is based on the overexpression of a potassium channel that regulates neuronal excitability. Potassium channels control the flow of potassium ions in and out of cells. Overexpression of potassium channels means enhanced regulation, leading to reduced cell activity and thereby preventing seizures.
“We are very excited to see that this novel gene therapy has the potential to be an effective alternative to surgical treatment for patients with focal cortical dysplasia,” said Professor Gabriele Lignani, co-corresponding author and a researcher at the Queen Square Institute of Neurology, University College London.
Gene therapy has previously been proven effective for another form of epilepsy occurring in the temporal lobe but had not been tested in focal cortical dysplasia. In this new study, the authors introduced an engineered potassium channel gene named EKC into the affected epileptic frontal lobe of mice. To increase safety, they used a non-replicating virus to carry the potassium channel gene. Before treatment, they monitored the brain activity of the mice for 15 days. They then injected the virus carrying the EKC gene or a control virus into the affected brain area. Subsequently, they monitored the brain activity of the mice for another 15 days.
They found that, compared to control mice, the gene therapy reduced seizures by an average of 87% in mice that received the therapy, without affecting their memory or behavior.
“After the success in mouse studies, we believe this therapy is suitable for clinical translation and, considering the scale of unmet needs, it could potentially be applied to thousands of children currently severely affected by uncontrolled epileptic seizures,” said Dr. Vincent Magloire, co-author and researcher at the Queen Square Institute of Neurology, University College London.
Professor Dimitri Kullmann, co-corresponding author and researcher at the Queen Square Institute of Neurology, University College London, added, “Plans for the first human clinical trials are underway, with completion anticipated within the next five years.”
Reference
1. Almacellas Barbanoj, Amanda, et al. “Anti-seizure gene therapy for focal cortical dysplasia.” Brain 147.2 (2024): 542-553.
