Recently, a CRISPR-Cas9 therapy for sickle cell disease has been approved, showcasing the potential of gene editing tools to effectively knock out genes for treating genetic disorders. However, the replacement of entire genes in the human genome to substitute defective or harmful genes remains unfeasible.
A new technology utilizing retrotransposons derived from birds to insert genes into the genome brings more hope for gene therapy. This technology allows the insertion of genes into “safe harbors” in the human genome, where inserted genes do not disrupt vital genes or cause cancer.
Retrotransposons are DNA segments that, when transcribed into RNA, encode enzymes that copy RNA into DNA in the genome—a self-serving cycle that fills the genome with retrotransposon DNA. Approximately 40% of the human genome consists of this “selfish” new DNA, though much of it has lost function, termed “junk DNA.”
This new technology, called “Precise RNA-mediated INsertion of Transgenes (PRINT),” harnesses the ability of certain retrotransposons to effectively insert entire genes into the genome without affecting other genomic functions. PRINT complements the capabilities of CRISPR-Cas technology in gene knockout, point mutations, and short DNA fragment insertion.
PRINT was developed by Professor Kathleen Collins’ laboratory at the University of California, Berkeley. Described in a paper published online in the journal Nature Biotechnology on February 20, 2024, titled “Harnessing eukaryotic retroelement proteins for transgene insertion into human safe-harbor loci.”
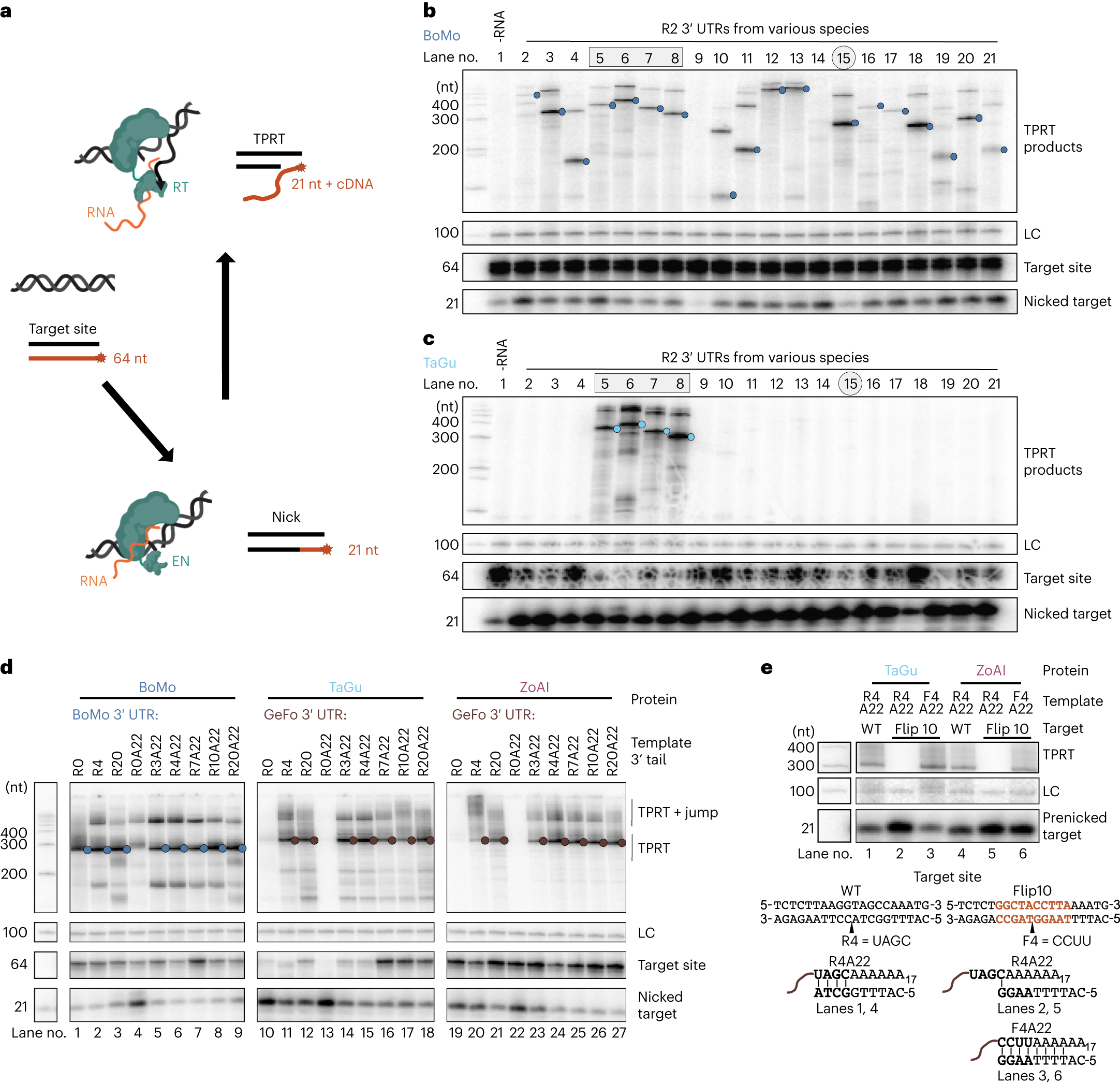
PRINT involves using a delivery method similar to delivering CRISPR-Cas9 into cells for genome editing to insert new DNA into cells. In PRINT, a delivered RNA encodes a common retrotransposon factor called the R2 protein, which contains multiple functional domains, including a nickase—an enzyme that binds to and cuts double-stranded DNA—and a reverse transcriptase—an enzyme that uses DNA as a template to produce RNA. Another RNA acts as a template for inserting transgenic DNA, along with gene expression control elements, forming an entire autonomous transgenic cassette in the genome.
One key advantage of using the R2 protein is its ability to insert transgenes into regions of the genome containing hundreds of copies of the same gene—each copy encoding ribosomal RNA, which is the RNA machinery translating messenger RNA (mRNA) into proteins. With so many redundant copies, disruption of one or a few ribosomal RNA genes by the inserted gene does not have adverse effects.
Inserting transgenes into safe harbors avoids a major issue encountered when inserting transgenes via viral vectors into the genome: genes are often randomly inserted, leading to loss of function in working genes or disruption of gene regulation or function, potentially resulting in cancer.
Collins stated, “CRISPR-Cas9-based methods can fix a mutated nucleotide or insert a small DNA sequence. Or you can knock out gene function through site-specific mutagenesis. We’re not knocking out gene function. We’re not fixing endogenous gene mutations. We’re taking a complementary approach by adding a gene into the genome that can autonomously express a protein, serving as a missing bypass, re-adding a functional gene. This is transgenic supplementation, not gene mutation reversal. This is a very good approach for repairing loss-of-function diseases caused by a series of individual mutations in the same gene.”
Many genetic diseases, such as cystic fibrosis and hemophilia, are caused by multiple different mutations in the same gene, all of which result in loss of gene function. Any CRISPR-Cas9-based gene editing therapy must be tailored to the specific mutations of the patient. With PRINT for gene supplementation, the correct genes can be delivered to each patient, allowing each patient’s body to produce normal proteins regardless of the original gene mutations.
Many academic labs and startups are researching how to use transposons and retrotransposons to insert genes for gene therapy. A popular retrotransposon being studied by biotech companies is LINE-1 (Long INterspersed Element-1), which can replicate itself and some hitchhiking genes in humans, occupying about 30% of the entire genome, although fewer than 100 copies of LINE-1 retrotransposons in the human genome are functional, constituting a tiny fraction of the genome.
Collins, along with her postdoctoral colleague Akanksha Thawani and Professor Eva Nogales from the University of California, Berkeley, published the low-temperature electron microscopy structure of LINE-1-encoded enzyme protein in Nature on December 14, 2023 (Nature, 2023, doi:10.1038/s41586-023-06933-5).
Collins said that this new research clearly indicates that LINE-1 retrotransposon proteins have difficulty in effectively and safely inserting transgenes into the human genome through genetic manipulation. However, previous studies have shown that genes inserted into the repetitive ribosomal RNA coding regions (rDNA) of the genome can be expressed normally, which led Collins to consider another retrotransposon factor named R2, which might be better suited for safe gene insertion.
Since R2 does not exist in the human body, Collins and her senior researcher Xiaozhu Zhang and postdoctoral researcher Briana Van Treeck from the University of California, Berkeley, screened R2 from dozens of animal genomes ranging from insects to horseshoe crabs and other multicellular eukaryotes to find a version highly targeted to the rDNA region in the human genome and efficiently insert long DNA into that area.
Collins said, “After searching dozens of species, the real winners came from birds,” including zebra finches and white-throated sparrows. She noted that while mammals lack R2 in their genomes, they have binding sites necessary for R2 as a retrotransposon factor to insert effectively—suggesting that mammals’ ancestors might have had a R2-like retrotransposon factor, which was somehow purged from mammalian genomes.
In experiments, Zhang and Van Treeck synthesized mRNA encoding the R2 protein and a template RNA producing a transgene carrying a fluorescent protein, whose expression is controlled by an RNA polymerase promoter. These were co-transfected into human cells cultured in vitro. Under laser irradiation, about half of the cells lit up green or red due to expression of the fluorescent protein, indicating successful insertion of the working fluorescent protein by the R2 system into the genome.
Further studies showed that the transgene indeed inserted into the rDNA region of the genome, and approximately 10 copies of the RNA template could be inserted without disrupting the protein-making activity of the rDNA genes.
Besides providing safe harbors, inserting transgenes into the genome’s rDNA region offers other benefits. The rDNA region is located on the short arms of five independent chromosomes, all packed together to form a structure called the nucleolus, where DNA is transcribed into ribosomal RNA and folded into the ribosomal machinery for protein synthesis.
Within the nucleolus, transcription of rDNA is highly regulated, and its genes are rapidly repaired, as any breaks in rDNA, if left unchecked, can halt protein production. Therefore, any transgenes inserted into the genome’s rDNA region are tightly protected within the nucleolus.
Collins said, “The nucleolus is a huge ribosome biogenesis center. But it’s also an extremely privileged DNA repair environment, with
a low risk of carcinogenesis from gene insertion. These successful retrotransposons entering the nucleolus DNA are really remarkable. It’s multicopy, it’s conservative, it’s a safe harbor, you can destroy one copy, and the cell doesn’t care.” This makes the region an ideal location for inserting genes for human gene therapy.
Collins acknowledges that there are still many unknowns about the mechanism of R2 and biological questions about rDNA transcription: how many rDNA genes will be disrupted before the cell takes notice? Since some cells shut down many genes from the 400+ rDNA genes in the human genome, are these cells more susceptible to the side effects of PRINT?
She and her team are researching these questions while also tweaking various proteins and RNAs involved in retrotransposon insertion to make PRINT more effective in cells cultured in vitro and primary cells from human tissues.
Reference
1. Zhang, Xiaozhu, et al. “Harnessing eukaryotic retroelement proteins for transgene insertion into human safe-harbor loci.” Nature Biotechnology (2024): 1-10.
