Researchers from Charité-Universitätsmedizin Berlin, Berlin Institute of Health and Deutsches Rheuma-Forschungszentrum, together with other scientists, have shown how microbes help the immune system respond to pathogens. Without the relevant mediators, the metabolic processes of some immune cells cannot be activated.
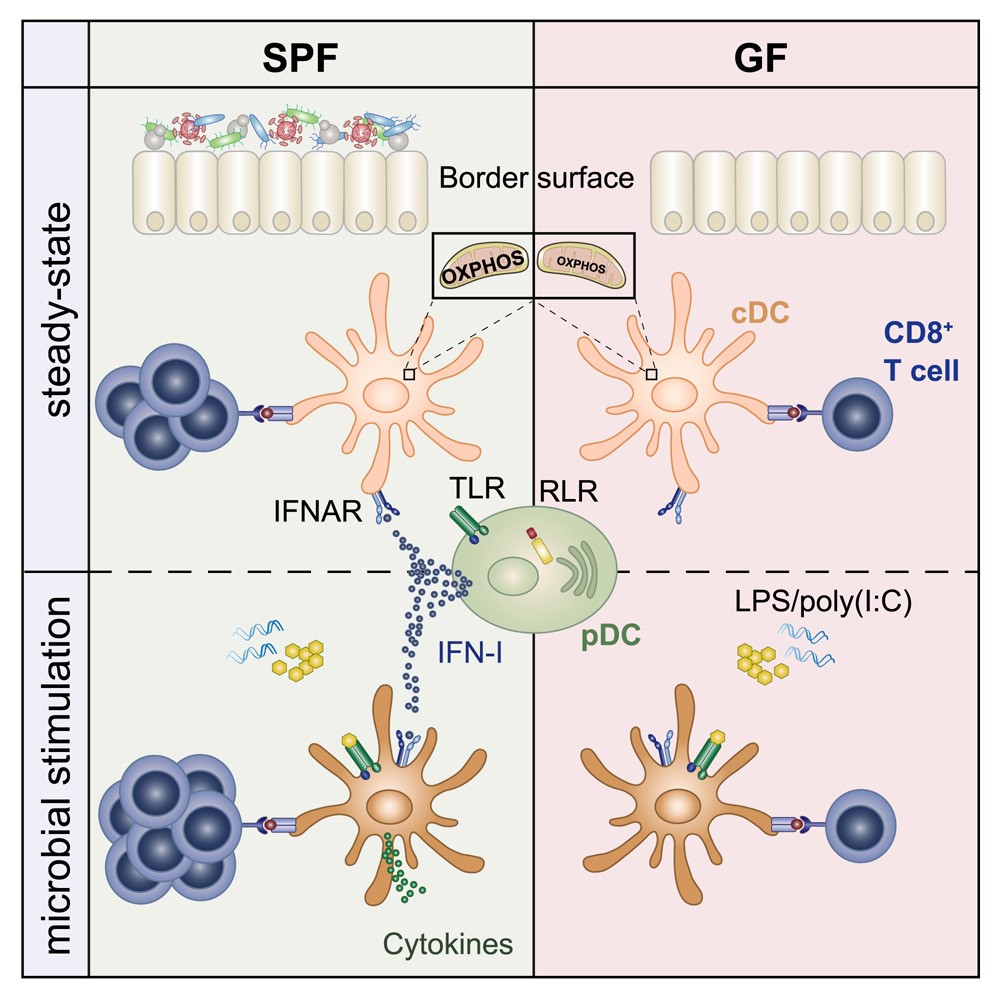
Human epithelial tissue is located at the environmental interface and is a potential channel for pathogen invasion. These tissues are also naturally colonized by complex communities of bacteria, viruses, fungi and parasites, known as microbial communities. In the course of evolution, permanent interactions with these microbes are likely to lead to the development of robust signaling pathways, which help protect the body. A research team led by Professor Andreas Diefenbach has been studying the role of microbiome in the body’s immune response to harmful pathogens and its impact on signaling pathways.
Infection could activate the body’s immune response. In this process, conventional dendriticcells (cDCs) plays a key role. They form part of the body’s innate immune system and carry a series of pattern recognition receptors that enable them to quickly identify invading pathogens. The cell’s initial response includes the release of cytokines, a signaling protein that attracts immune cells to the infected site. At the same time, these cells also engulf and digest invading pathogens through phagocytosis, and then present individual particles in the form of antigens on the cell surface. This in turn leads to the activation of T cells and leads to targeted immune responses. On the contrary, when cDCs provides endogenous antigens to trigger T cell activation, this can lead to wrong and adverse immune responses and lead to autoimmune diseases.
The researchers used a variety of animal models to learn more about how microbial-controlled IFN-I starts ground-state cDCs. Using sequencing techniques, the researchers were able to compare the epigenome and transcriptome of cDCs from aseptic animals, control animals and IFN-I receptor deficient animals. The researchers want to know what happens at the molecular level when cDCs is no longer exposed to IFN-I. “Interestingly, when we looked at the cDCs of aseptic animals and those that did not have IFN-I signals, we were able to observe low levels of expression of genes involved in the mitochondrial respiratory chain,” Laura Schaupp, lead author of the study, said in describing the observations. “Further analysis shows that the metabolism of cDCs cells from sterile animals is abnormal, preventing them from initiating an immune response. These cells actually lack the fuel needed to respond to pathogens.” This indicates that microbiome plays an important role in the function of cDCs. It seems to be critical to the ability of cDCs to respond effectively to bacterial or viral infections, including T cell-mediated responses.
The researchers’ findings may help to develop new treatments. Many autoimmune diseases, such as systemic lupus erythematosus, are caused by an increase of IFN-I. Other studies have shown that microbiome affects the effectiveness of immune checkpoint inhibitors in cancer immunotherapy.