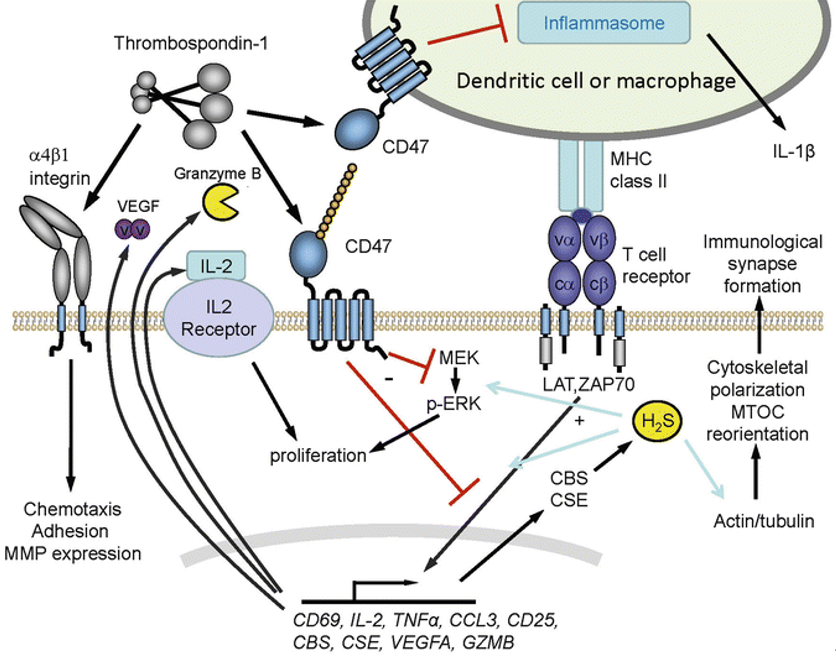
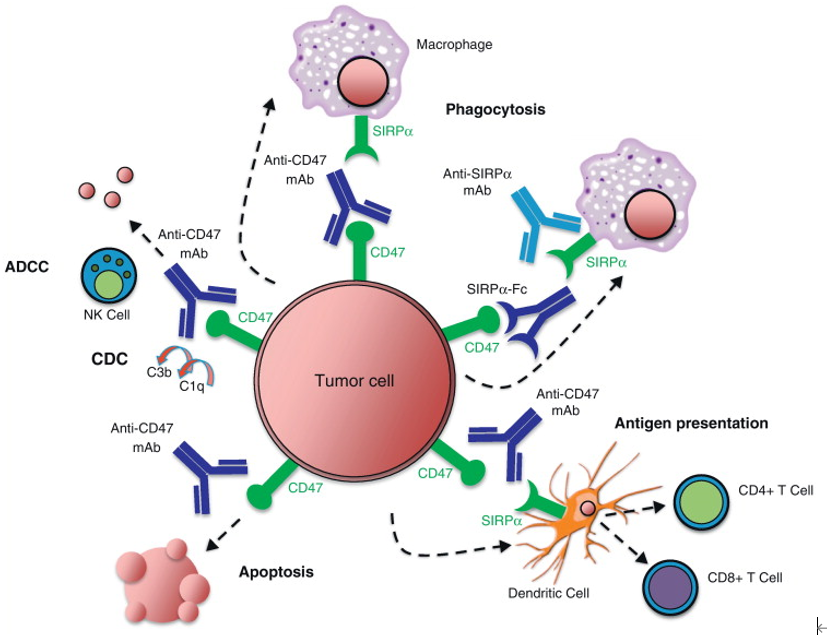
2 CD47-related Signaling Pathways
The extracellular ligands interacting with CD47 are TSP1, SIRP α, and CD47 itself. Among them, little is known about the signal transduction involved in the homotypic interaction of CD47 itself. Therefore, at present, the main signaling pathways involved in CD47 can be roughly divided into two streams according to its ligands, that is, the signaling pathways regulated by TSP1 or SIRPα, as shown in figure 2.
2.1 CD47/TSP-1 Signaling Pathway
2.1.1 Regulation of G-protein Pathway
The complex formed by CD47 and integrin can interact with trimeric G-protein through its intracellular ligand PLIC1 to form CD47-integrin-G-protein complex. The five-transmembrane domain of CD47 and two-transmembrane fragment of integrin form a temporary seven-transmembrane complex (7TMS) that can activate G-protein. Different integrin ligands and CD47 isomers with different cytoplasmic tails can endow 7TMS complex the function of activating specific trimeric G-protein. G-protein can mediate a variety of cellular effects of CD47, such as CD47-related platelet activation requires G-protein to activate Syk kinase, thus inducing phosphorylation of Lyn and FAK. Another component of the CD47-integrin-G-protein complex is cholesterol. Cholesterol removal does not affect integrin affinity but reduces the ability of CD47 to stimulate cell adhesion to hyaluronectin. Therefore, although the basic cellular diffusion mechanism has nothing to do with cholesterol, cholesterol seems to play a necessary role in maintaining the functional CD47-integrin-G-protein complex.
Fig. 2. Schematic diagram of CD47/TSP-1 signaling pathways. (SpringerLink)
2.1.2 Regulation of NO/cGMP Signaling Pathway
Endothelial NO synthase (eNOs) is the main synthase isomer of nitric oxide (NO) in vascular cells, which can convert L-arginine to L-citrulline and release NO. eNOs activity is regulated by mechanical stimulation and circulatory factors such as VEGF and acetylcholine. VEGF activates PI3K through VEGFR2 on endothelial cells, which activates protein kinase B (PKB, also known as Akt). Akt activates eNOs by phosphorylating serine 1177. At the same time, activated VEGFR2 recruits Src and induces Ca2+ signaling pathway to further activate eNOs. The resulting NO binds to the heme structure of soluble guanylatecyclase (sGC) in target cells, increases its enzyme catalytic activity, leads to intracellular cGMP accumulation, and activates cGMP-dependent protein kinase (cGK1), which leads to dephosphorylation of myosin light chain and relaxation of smooth muscle. TSP1 can bind to CD47, destroy the complex of CD47 and VEGFR2, inhibit the activation of VEGFR2, and then affect the downstream signaling pathway.
2.1.3 Regulation of Cell Survival
The combination of TSP1 and CD47 can induce the transfer of BNIP3 to mitochondria. The transmembrane domain of BNIP3 can insert into the mitochondrial membrane, causing the mitochondrial permeability transition pore to open and release cytochrome C, resulting in cell death. Lack of CD47 helps cells promote their survival by activating protective autophagy pathways. Autophagy is a catabolic mechanism to maintain the stability of the internal environment, which is a process in which cytoplasmic sols and organelles are isolated into vesicles of bilayer membranes and transported to lysosomes/vacuoles for degradation, resulting in the recycling of macromolecules. Mild autophagy can prevent cells from being damaged by adverse factors and help them survive. When CD47-deficient cells were exposed to radiation, increased expression of beclin-1, ATG5, ATG7 and LC3, decreased expression of p62, and increased formation of autophagosomes can activate the protective autophagy. Among them, Beclin-1 can also bind to Bcl-2/Bcl-xL to form a complex, so the activation process of autophagy will be inhibited. Therefore, the interaction between Beclin-1 and Bcl-2 can directly control autophagy and apoptosis.
2.2 D47/SIRPα Signaling Pathway
2.2.1 Regulation of Phagocytosis
As an autologous recognition molecule, CD47 can interact with SIRPα on the surface of macrophages to prevent tumor cells and red blood cells from being cleared. This mechanism of preventing macrophage phagocytosis involves the inhibitory signaling pathway of SIRPα, which is partly through SHP-1 to inhibit macrophage phagocytosis.
In addition, some studies have found that the function of CD47-SIRPα interaction in erythrocyte clearance may be more complex. The senescence of human red blood cells seems to be related to the conformational change of CD47, which may rely on oxidative modification, and can change the structure of molecules from “don’t eat me” to “eat me”. Therefore, CD47 is not only a “don’t eat me” signal but may also be used as a switch to regulate the clearance of red blood cells. The abnormal expression of CD47 or SIRPα or the interference of CD47-SIRPα interaction will change the phagocytosis of macrophages and destroy the homeostasis of red blood cells.
Fig. 3. Schematic diagram of CD47/SIRPα signaling pathways. (Current Opinion in Immunology)
2.2.2 Regulation of In Vivo Homeostasis of Dendritic Cells and T Cells
Some studies showed that in the spleen of SIRPα mutant mice, the number of CD4+ Dendritic cells (DCs), the half-life of CD4+ DCs, and the number of CD4+ T cells all decreased, and the T cell aggregation area became smaller. It is suggested that the interaction between CD47 and SIRPα plays an important role in regulating the homeostasis of DCs and T cells in vivo. In addition, some studies have found that DCs and T cell responses are affected by the interaction between CD47 and SIRPα at many levels. Interference with the interaction between CD47 and SIRPα can impair the function of DCs, especially in inducing responses of helper T cells (Th1, Th2 and Th17) and natural killer T cell.