Introduction of Immunogenicity
In the description of this overview, immunogenicity is defined as the propensity of the therapeutic biologics to generate immune responses to itself and to related proteins or to induce immunologically related non clinical affect or adverse clinical events. There are two types of immunogenicity in therapeutic biologics development process: wanted immunogenicity and unwanted immunogenicity. Differentiation has to be made between them:
- Wanted immunogenicity is typically related with vaccines, where the injection of an antigen (the vaccine) stimulates an immune response against the pathogen (virus, bacteria, cancer cell…) aiming at protecting the organism.
- Unwanted immune responses to therapeutic biologics may also neutralize their biological activities and result in adverse events not only by inhibiting the efficacy of the therapeutic biologics, but also by cross-reacting to an endogenous protein counterpart, leading to loss of its physiological function (e.g., neutralizing antibodies to therapeutic erythropoietin cause pure red cell aplasia by also neutralizing the endogenous protein). The meaning of immunogenicity in this overview is the latter adverse immune response in the therapeutic biologics discovery and development.
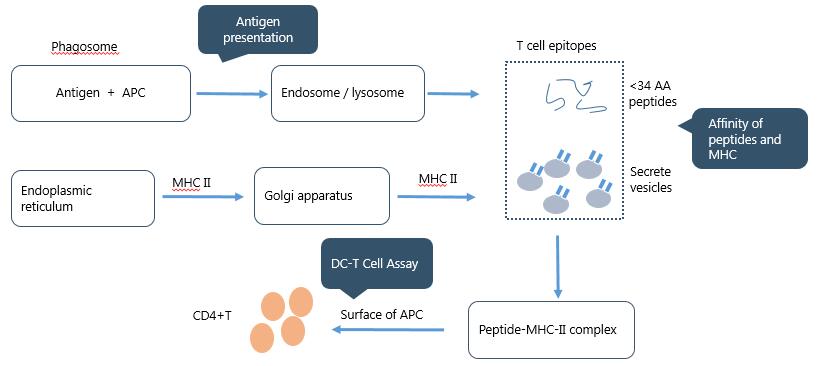
Antigen processing and presentation plays a critical role in immunogenicity of organisms. The processes are performed by professional APCs (antigen presentation cells) such as DCs, macrophages and B cells. There are two involved events in the process: 1. antigen capture that delivers antigens to the cellular antigen processing machinery; 2. antigen processing and presentation that generates antigenic peptides bound to MHC molecules for presentation to adaptive immune cells. The detailed process is illustrated here: 1. Extracellular antigens are captured by APCs through phagocytosis, macropinocytosis and receptor-mediated endocytosis. In acidic environment of endosome or lysosome, antigens are degraded into many immunogenic peptides which contain T cell epitopes. Following intake of antigens, MHC class II molecular is synthesized in the endoplasmic reticulum, and then transported by golgi apparatus to combine with antigen peptides to form peptide-MHC II complexes. 2. The complexes are presented to the surface of APCs. TCR can recognize the peptide-MHC II complexes to activate T cells to initiate an immunogenic response. The quality of the antigen presentation depends on affinity of the peptide-MHC complexes and there is a direct relationship between peptide-MHC complex stability and the immunogenic response. In addition, binding ability between APCs and CD4+ T cell is determined by DC-T cell assay (Fig. 1).
Fig. 1 Background of Immunogenicity
Importance of Immunogenicity in Therapeutic Biologics Development
Therapeutic biologics, including protein, enzyme, antibody, antibody-drug conjugate (ADC), are of significant value in the treatment of various diseases. “What about immunogenicity?” This is often the first question asked when a discussion about therapeutic biologics development occurs. Thus this issue has been the subject of a wide variety of reference material, and has been the subject of a cross-industry group that has written multiple white papers on aspects of immunogenicity. Immune responses to therapeutic biologics may pose problems for both therapeutic biologics efficacy and patient safety patient safety. The first generation therapeutic mAbs were of murine origin, leading to highly adverse immune responses in patients because of the foreignness of the antibodies. The adverse immune response, also named immunogenicity, led to the production of anti-drug antibodies (ADAs), which resulted in enhanced clearance of the drug and other safety implications. Immunologically based adverse events, such as anaphylaxis, cytokine release syndrome, and cross-reactive neutralization of endogenous proteins mediating critical functions, have caused sponsors to terminate the development of what otherwise may have been efficacious therapeutic biologics. Except for these real cases, effect of immunogenicity in the therapeutic biologics development can be summarize as follows:
- Effects on bio-availability
- Effect on safety and efficacy
- Effect on PK including potential cross reactivity to endogenous proteins
- Inhibition of the function of endogenous protein
- Injection site reactions
- Systemic reactions mild or life threatening
- Formation of ADA (HAMA, HACA, HAHA)
- Formation of neutralizing antibodies
- Formation of immune complexes
- Formation of anti-idiotypic antibodies
Fundamentally, with therapeutic biologics being developed today, the most important factor concerning immunogenicity is that it is a co-variate of pharmacokinetics, when immunogenicity against a therapeutic biologics occurs, it increases clearance and decreases exposure to that therapeutic. Both patient-related and product-related factors may affect immunogenicity of therapeutic biologics. These factors are critical elements in the immunogenicity risk assessment. Ideally, these factors should be taken into consideration in the early stages of therapeutic biologics development. It is a vital and necessary path to assess immunogenicity in biopharmaceutical drug candidates screening process.
Immunogenicity Assessment Strategy
Immunogenicity assessment is one of the regulatory requirements for therapeutic biologics approval which includes the review of immunogenicity studies and the interpretation of the results. As stated in authoritative guidelines on therapeutic biologics, immunogenicity should be investigated in the target population since animal testing and in vitro models cannot predict immune response in humans. In addition, immunogenicity has a role in demonstrating product comparability following manufacturing changes and similarity in the context of biosimilar development. Even minor changes can potentially affect the bioactivity, efficacy or safety including immunogenicity of a therapeutic biologic.
Characterisation and screening for physico-chemical determinants or formulation-based factors will aid both in the prediction of immunogenicity and in the development of less immunogenic therapeutic agents, such as impurities, heterogeneity, aggregate formation, oxidation and deamidation in the therapeutic biologics. Moreover, predicting potential immunogenic epitopes in therapeutic biologics will be an important and effective strategy to improve their safety and efficacy. A variety of preclinical immunogenicity assessment strategies are being used during therapeutic biologics development as listed in Table 1.
Table 1 Strategies in predicting and reducing immunogenicity to therapeutic biologics
| Prediction | Reduction |
| Physiochemical characterization | Deimmunization (epitope modifications) |
| In Silico immunogenicity assessment | Humanization |
| T cell epitope predictions | |
| B cell epitope predictions | |
| In Vitro immunogenicity assessment | Purity and formulations |
| Ex Vivo immunogenicity assessment | Purity and formulations |
| T cell response Modifications | |
| HLA binding assays | Fusion proteins |
| In Vivo immunogenicity assessment | Combination biologics or combination therapy |
For many antibody drug or biosimilar, anti-drug antibodies (ADAs) has been generated in preclinical or clinical studies, resulting in adverse impact efficacy of therapeutic biologics. Anti-drug antibodies (ADAs) assay becomes an indispensable part in immunogenicity assessment for therapeutic drug candidate screening. Following current FDA and EMEA guidelines and AAPS white papers, Creative Biolabs is an experienced expert on ADA assay development and validation.
The consequences of immune responses to therapeutic biologics can range from no apparent effect to serious adverse events, including life-threatening complications such as anaphylaxis, neutralization of the effectiveness of lifesaving or highly effective therapies, or neutralization of endogenous proteins with nonredundant functions. Therefore, a systematic evaluation of immunogenicity is necessary for approval of all therapeutic biologics and biosimilar medicines. Assessment of immunogenicity should be included in post-marketing monitoring and considered in risk management and risk mitigation plans for all therapeutic products. With such safeguards and pharmacovigilance in place, the risk of immunogenicity of a biotherapeutic should be minimized resulting in better and safer products.