Human immunodeficiency virus 1 (HIV-1) is a retrovirus, and most HIV-1 infections occur on the mucosal surface of the human reproductive tract. After HIV-1 enters the nucleus of the host cell, it integrates its own nucleic acid sequence into the DNA of the host cell and establishes infection within days to weeks. However, the mechanism by which HIV-1 enters the host cell nucleus is not fully understood. A study in the journal Nature Communications has discovered a new mechanism by which HIV-1 enters the host cell nucleus. In addition, the researchers also identified three proteins required for HIV-1 to invade host cells and synthesized a drug molecule that can target one of the proteins. This discovery is expected to explore new treatments for AIDS.
How extracellular vesicles (EVs) enter cells
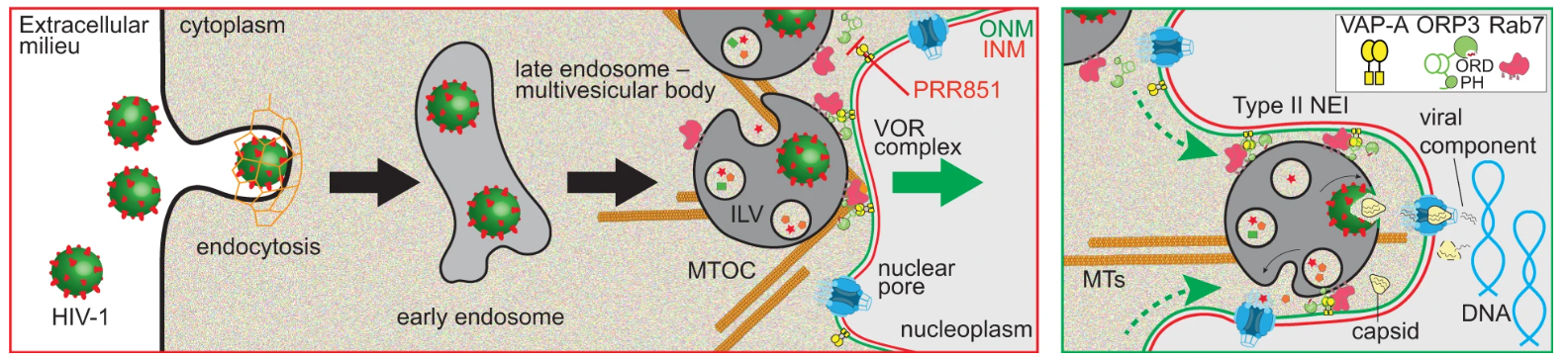
Endocytosed EVs enter the nucleoplasmic reticulum (NR), especially type II nuclear envelope invaginations (NEIs); then pass through nuclear pore complexes (NPCs) on the nuclear membrane to deliver EVs to the interior of the nucleus. The premise of this process is that late endosomes dock on the nuclear outer membrane. This docking process is mediated by the VOR protein complex. The docking is mediated by the VOR protein complex, comprised of ONM-localized vesicle-associated membrane protein (VAMP)-associated protein A (VAP-A), cytoplasmic oxysterol-binding protein (OSBP)-related protein-3 (ORP3), and late endosome-associated small GTPase Rab7.
The Trojan exosome hypothesis points out that retroviruses can use intercellular vesicles for communication and exosomes for transport to generate retroviral particles and then infect normal cells (exosomes are a type of extracellular vesicles). The researchers speculate that the exosome pathway has similarities to the viral life cycle. The researchers found that, analogous to the entry method of extracellular vesicles, “camouflage” HIV-1 on vesicular stomatitis virus G (VSV-G) protein or native Env protein, and after being internalized into HeLa cells or primary CD4+ T cells, its contents can be delivered into the host cell nucleus through the same pathway as EVs.
Fig. 1 Representation of the induction of type II NEIs by virus-laden late endosomes. (Santos, M.F., 2023)
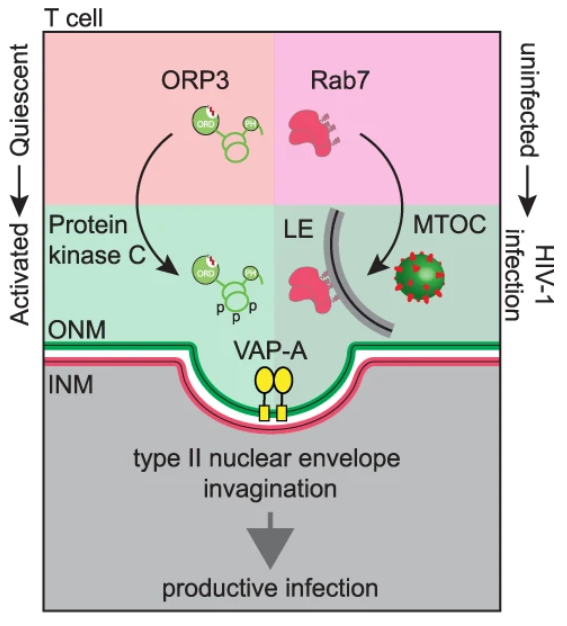
The researchers also found that, similar to the process of extracellular vesicles entering the cell, three proteins are critical for HIV-1’s nuclear import process. The first protein Rab7 is located on the membrane of the endosome, the second protein VAP-A is located on the inverted nuclear membrane, and the third protein ORP3 links the first two proteins together. Successful HIV-1 entry requires an interaction between three proteins, so targeting any one of them can block infection. The researchers synthesized and tested molecules that block interactions between proteins. The data showed that in the presence of these molecules, HIV’s replication process was inhibited.
Fig. 2 Representation of type II NEI induction that requires both T cell activation leading to ORP3 hyperphosphorylation (left) and viral infection leading to the accumulation of Rab7+ late endosomes (LE) at the microtubule-organizing center (MTOC, right). (Santos, M.F., 2023)
Professor Aurelio Lorico, the corresponding author of the paper, said, “Although our research is still in the preclinical stage, the new drugs synthesized are likely to have therapeutic effects on AIDS, other viral diseases, metastatic cancer, and other diseases involving nuclear transport.” The team is currently investigating the role of this pathway in Alzheimer’s disease and in the metastasis of various cancers.
Reference
Santos, M.F., Rappa, G., Karbanová, J. et al. HIV-1-induced nuclear invaginations mediated by VAP-A, ORP3, and Rab7 complex explain infection of activated T cells. Nat Commun 14, 4588 (2023). https://doi.org/10.1038/s41467-023-40227-8