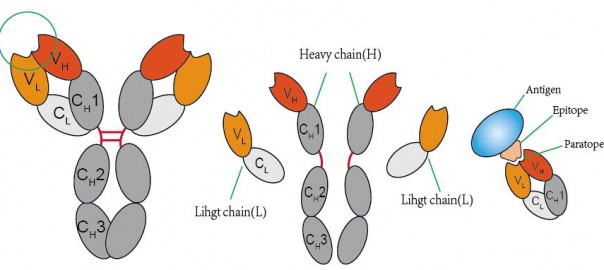
Application of Single Domain Antibodies in Cell Therapy
G protein-coupled receptor (GPCR) is a very important receptor protein on the cell surface. In the human genome, GPCR-encoding genes account for more than 2% and contain more than 800 members, makingRead More…