Single domain antibodies have been widely used as antigen-binding domains in CAR-T due to their small size, excellent stability, high affinity, and strong manufacturing feasibility. Single domain antibody-based CAR constructs have shown promising functions on more than a dozen different tumor-specific targets. The resulting single domain antibody-based CAR-T or CAR-NK cells have shown antitumor effects both in vivo and in vitro. The application of single domain antibodies in CAR-T therapy has been well-documented from the laboratory to the bedside and is showing great potential in forming more challenging advanced CAR-T.
A Brief History of CARs
Chimeric receptors were first proposed by Zelig Eshhar and Gideon Gross in 1989. They replaced the Vα and Vβ regions of the αβ-T cell receptor (TCR) with the VH and VL of the antibody, respectively, to construct an artificial chimeric TCR in the form of VH-Cα/VL-Cβ or VL-Cα/VH-Cβ. This chimeric TCR can bind target cells in an MHC-independent manner and activate cells through the TCR mechanism.
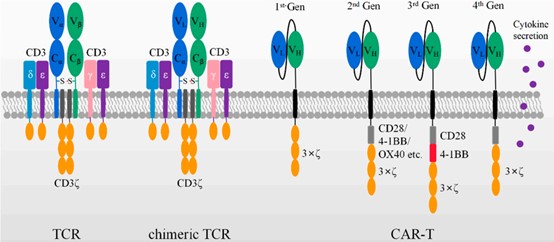
Figure 1. Schematic diagram of TCR, chimeric TCR, and different generations of scFv as CAR domains
The currently used CARs are composed of four components: an extracellular antigen recognition domain (including a single-chain antibody fragment scFv or single domain antibody VHH); structural components, such as hinge and transmembrane domains; co-stimulatory signaling domains that provide maintenance of CAR-T cell effector functions; and CD3ζ activation domains.
The first-generation CARs contained only the CD3ζ chain signaling domain. Although the first generation of CAR-T cells can specifically kill targeted tumor cells in vitro and in mouse models. However, tumor cells rarely express co-stimulatory receptor ligands such as B7, and the first-generation CARs did not have co-stimulatory receptor domains. As a result, they showed low cell killing activity and short persistence in vivo, and did not have clinical effectiveness.
The second-generation CARs were inserted with co-stimulatory domains, such as CD28, 4-1BB, or OX40, between the transmembrane sequence and the ITAM domain of CD3ξ. The second-generation CAR-Ts exhibited optimized T cell activation, increased antigen-dependent proliferation in vitro, enhanced persistence in vivo, and more potent antitumor activity. The second-generation CARs with CD28 or 4-1BB co-stimulatory domains are currently well established, and three FDA-approved anti-CD19 CAR-T cells are designed based on this structure.
The third-generation CARs were added with additional co-stimulatory domains to further enhance T cell activation. Typical intracellular domains consisted of CD28/4-1BB/CD3ξ or CD28/OX40/CD3ξ. Clinical trials showed that compared with the second-generation CARs, CAR-T cells carrying the third-generation CARs exhibited stronger expansion and longer survival time, especially in patients with mild disease and lower levels of normal B cells .
The fourth-generation CARs were added with the ability to secrete cytokines or antibodies, as well as enhanced anti-tumor activity by regulating the tumor immune microenvironment. The fourth-generation CAR-T cells combined direct anti-tumor function with the immunomodulatory ability to release cytokines at the tumor site, avoiding the adverse effects of cytokines caused by systemic administration, and they were expected to be used in the targeted therapy of solid tumors.
The fifth-generation CAR is designed based on the second-generation CAR, adding cytokine receptors to its intracellular domain.
Advantages of Replacing scFv with Single Domain Antibody As CAR Targeting Domain
No Aggregation Risk
ScFv, as a CAR, is easy to accumulate on the cell surface to trigger the activation of effector cells and a cytotoxic signaling cascade, leading to T cell exhaustion. The main reasons for the high self-aggregation tendency of scFvs are exposed free hydrophobic residues on the weight variable domains, and unstable folding of the VH/VL regions. In contrast, VHH-based CAR-T cells do not have this risk.
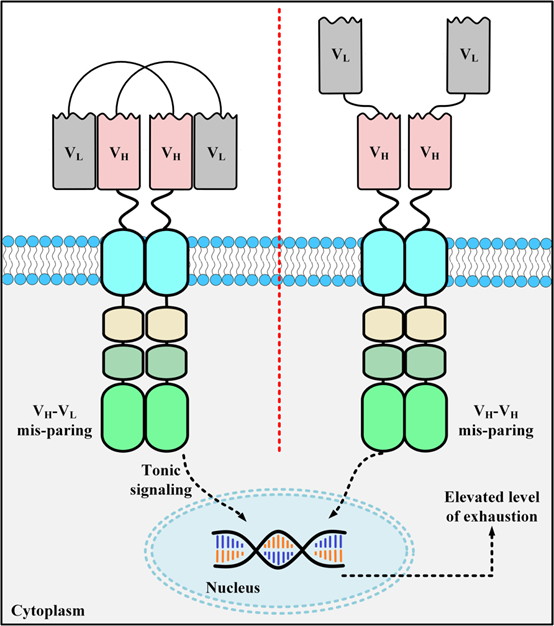
Figure 2. Schematic diagram of scFv aggregation on the surface of CAR-T
Low immunogenicity
The linker peptide sequence linking VH and VL in the scFv structure, as well as the backbone sequence of the murine antibody, can lead to immunogenicity risks, leading to the generation of anti-drug antibodies (ADA) in vivo. The ADA effect may neutralize the function of CAR-T cells, causing severe side effects, the loss of CAR-T cells, and even the failure of CAR-T therapy. In contrast, VHH does not require linker peptides, and the sequence similarity of VHH to human VH (VH3 gene family) is as high as 75-90%, so the risk of immunogenicity is unlikely to occur.
Bispecific CAR
The structure of scFv limits the potential to construct more complex CARs. When a bispecific CAR is constructed, the cross-pairing of VH and VL in two independent scFv molecules may lead to reduced affinity, and the size of multiple scFv inserted genes affects the viral packaging efficiency. VHH is more favorable than scFv, which is prone to be mismatched, to be constructed as the targeting domain of bispecific CAR, and the efficiency of transfection and virus packaging is also better than that of scFv.
In addition to the above advantages, VHH also has a more favorable structure in terms of antigen epitope binding, solubility, and stability. Due to these properties, VHHs have a unique potential for developing various forms of CAR-T.
Research Progress of Single Domain Antibodies in the Field of CAR-T Therapy
Single Domain Antibody-Based CAR
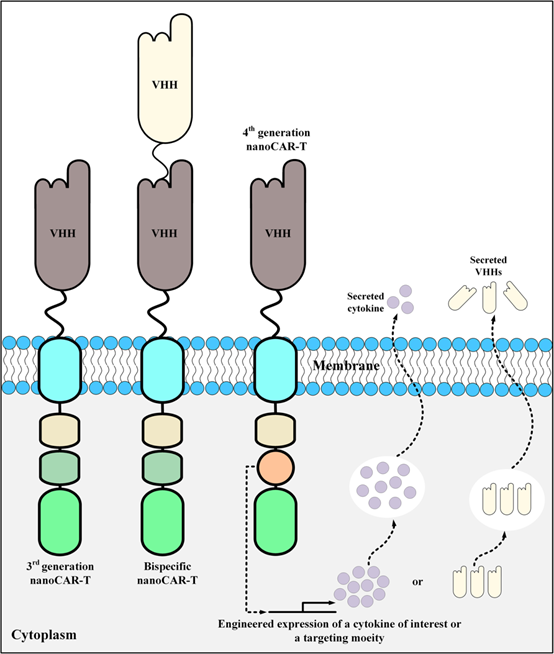
Figure 3. Schematic diagram of CAR-T design with single domain antibody as targeting domain
BCMA is a transmembrane activator and calcium regulator that plays an important role in the maturation and differentiation of B cells into plasma cells. Because malignant plasma cells express high levels of BCMA, they are an important target for immunotherapy of various cancers. Cilta-cel was approved by the FDA in 2022 for the treatment of adult relapsed/refractory multiple myeloma (MM). It is the first FDA-approved CAR-T in China and the world that uses a single domain antibody as the targeting domain. Cilta-cel adopts a unique bivalent single domain antibody design, and its approval for marketing shows that it is feasible to use single domain antibodies to develop and manufacture bispecific/bivalent CAR-T cells.
In addition to the approved Cilta-cel using a single domain antibody as the CAR-T targeting domain, current research on the CAR-T targeting domain of single domain antibodies has involved many popular targets, including VEGFR2, HER2, PSMA, TAG-72, GPC2, CD38, CD33, CD7, MUC1, EGFR, CD20, CD105, PD-L1, and EIIIB.
Single Domain Antibody-Based Targeting Modules
UniCAR T cells are an improved T cell therapy based on CAR technology. Unlike traditional CAR T cells, UniCAR T cells do not directly bind to tumor cells, but are redirected to specific cell surface antigens by binding to targeting modules. Due to the small size of single domain antibody molecules, they can penetrate tumor tissues more easily, and their preparation and synthesis are more flexible, so they are a good choice for targeting modules. Research data also demonstrated that bivalent VHH-based targeting modules can redirect UniCAR-expressing T cells to cancer cells expressing low levels of EGFR antigen.
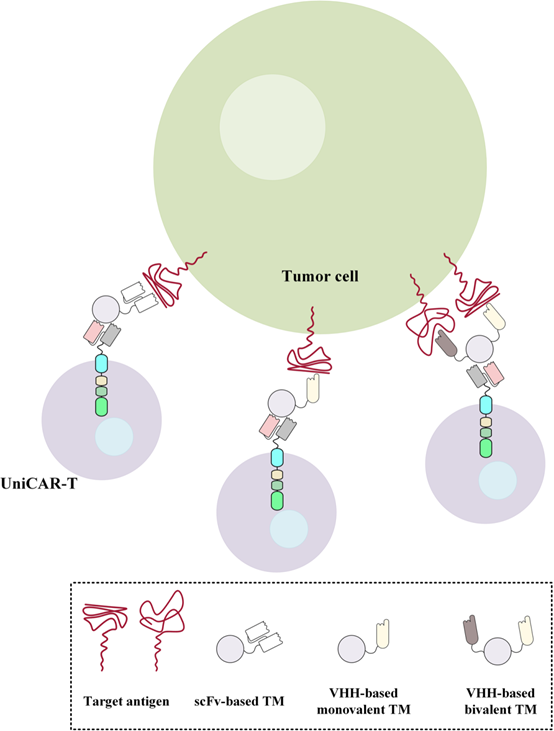
Figure 4. Redirection of UniCAR-expressing T cells to target specific antigens of interest using scFv, monovalent VHH, and bivalent VHH-based targeting modules
CAR-T with Autocrine Single Domain Antibody
At present, a number of CAR-T drugs have been approved for marketing in the world, but they are all targeting hematological tumors. While there are more patients with solid tumors, and the local microenvironment of tumors is more complex. The fourth-generation CAR-T is expected to regulate the tumor immune microenvironment of solid tumors through autocrine antibodies or cytokines to enhance its anti-tumor activity. Among them, the fastest progress is the autocrine PD1 single domain antibody MSLN-CAR T cell product (BZD1901). The drug is administered through intravenous infusion, and the reinfused CAR-T cells are activated after recognizing tumor antigens, killing tumor cells through cytotoxicity, and releasing PD1 single domain antibodies at the same time, which bind to PD1 on the surface of T cells to block PD- 1/PD-L1 signaling, change the inhibitory immune microenvironment, and exert a synergistic effect to kill tumor cells.
Single domain antibodies are an emerging and malleable tool in the field of cell therapy. Compared with traditional single-chain antibodies, single domain antibodies can more easily penetrate tumor cells, better recognize tumor targets, and produce anti-tumor effects. Single domain antibodies can also be used to construct multifunctional CAR-T cells, which can improve the efficacy and safety of treatment by targeting multiple targets. In addition, single domain antibodies can also be used as the targeting module of UniCAR-T to dynamically regulate and monitor CAR-T cells. With the deepening of its research, it is believed that the application of single domain antibodies in cell therapy will become more and more extensive.
Reference
Safarzadeh Kozani, Pouya, et al. “Nanobody-based CAR-T cells for cancer immunotherapy.” Biomarker Research 10.1 (2022): 24.
Bao, C., et al. “The Application of Nanobody in CAR-T Therapy. Biomolecules 2021, 11, 238.” (2021).
Ramos, Carlos A., et al. “In vivo fate and activity of second-versus third-generation CD19-specific CAR-T cells in B cell non-Hodgkin’s lymphomas.” Molecular Therapy 26.12 (2018): 2727-2737.
Albert, Susann, et al. “From mono-to bivalent: improving theranostic properties of target modules for redirection of UniCAR T cells against EGFR-expressing tumor cells in vitro and in vivo.” Oncotarget 9.39 (2018): 25597.
