In the dynamic realm of oncology, the integration of nanotechnology into cancer vaccines is emerging as a transformative frontier. These tiny particles, measuring between 1 and 100 nanometers, are revolutionizing the way we approach cancer immunotherapy by addressing long-standing challenges in targeted delivery, immune activation, and therapeutic efficacy. This article delves into the mechanisms, types, real-world applications, challenges, and future directions of nanoparticles in cancer vaccines, highlighting their potential to reshape cancer treatment.
Introduction to Nanoparticles in Cancer Immunotherapy
The Promise of Nanotechnology
Nanoparticles possess unique properties that make them ideal for enhancing cancer treatment. Their small size allows them to navigate through the body’s intricate vasculature, reaching even the most inaccessible tumor sites. Surface chemistry can be precisely tailored to conjugate with specific ligands, enabling them to target cancer cells or immune cells with high specificity. Moreover, they have the ability to encapsulate a wide range of therapeutic agents, including antigens, adjuvants, and drugs, protecting them from degradation and ensuring controlled release.
Cancer Vaccines 101
Cancer vaccines can be broadly classified into prophylactic and therapeutic types. Prophylactic vaccines, such as the HPV vaccine, aim to prevent cancer by stimulating an immune response against oncogenic viruses. Therapeutic vaccines, on the other hand, are designed to treat existing cancers by activating the body’s own immune system to recognize and attack cancer cells. They work by presenting tumor antigens to immune cells, particularly antigen-presenting cells (APCs), which then prime T cells to mount an antitumor response. The activation of long-term immune memory is a key feature of therapeutic vaccines, potentially leading to durable cancer control.
Why Nanoparticles?
Compared to traditional cancer treatment methods, nanoparticles offer several distinct advantages. Traditional vaccine delivery systems often suffer from poor targeting, leading to low accumulation in tumor sites and significant off-target effects. Nanoparticles, with their targeted delivery capabilities, can specifically deliver antigens and adjuvants to APCs, enhancing the efficiency of antigen presentation. They also enable sustained release of therapeutic agents, maintaining a prolonged immune stimulation that is crucial for mounting a robust antitumor response. Additionally, nanoparticles can modulate the immune system by influencing the polarization of immune cells, creating a more favorable immune microenvironment for cancer elimination.
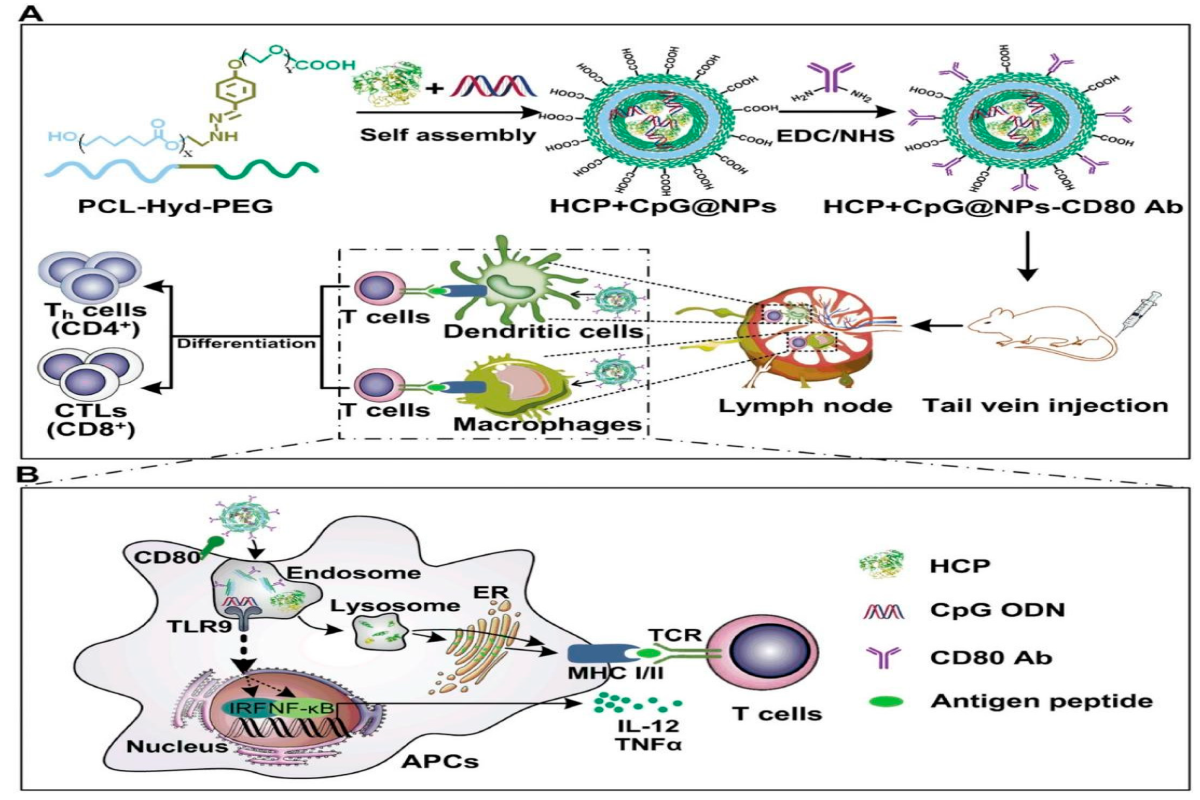
Fig.1 The mechanisms of EAC-NP-induced cancer immunotherapy.1
How Nanoparticle-Based Vaccines Work
Mechanisms of Action
- Antigen Delivery
One of the primary functions of nanoparticles in cancer vaccines is to protect antigens from degradation in the body. Antigens, whether they are peptides, proteins, or nucleic acids, are often vulnerable to enzymatic degradation when administered alone. Nanoparticles act as protective carriers, encapsulating antigens and delivering them to APCs, such as dendritic cells (DCs), in a stable form. Once taken up by DCs, the antigens are processed and presented on the cell surface in the context of major histocompatibility complex (MHC) molecules, initiating an immune response.
- Immune System Activation
Nanoparticle-based vaccines play a crucial role in stimulating dendritic cells, which are the master regulators of the immune system. By delivering antigens and adjuvants to DCs, nanoparticles induce DC maturation, upregulating the expression of co-stimulatory molecules and cytokines that are essential for T-cell activation. This leads to the proliferation and differentiation of antigen-specific T cells, including cytotoxic T lymphocytes (CTLs) that can directly kill cancer cells. Moreover, these vaccines help in establishing long-term immune memory, ensuring that the immune system can recognize and eliminate cancer cells if they reappear in the future.
Key Innovations
- Liposomal Nanoparticles
The success of mRNA vaccines in the COVID-19 pandemic has highlighted the potential of liposomal nanoparticles in antigen delivery. Liposomes are phospholipid-based vesicles that can encapsulate mRNA, protecting it from degradation and facilitating its uptake by cells. In cancer vaccines, liposomal nanoparticles are being used to deliver mRNA encoding tumor antigens, enabling the in situ production of antigens in APCs and triggering a strong immune response.
- Cancer Cell Membrane-Coated NPs
A novel approach in cancer vaccine development is the use of nanoparticles coated with cancer cell membranes. These nanoparticles mimic the surface characteristics of tumor cells, presenting a wide array of tumor antigens to the immune system. This targeted immune activation allows the immune system to recognize and attack cancer cells more effectively, while minimizing damage to normal cells.
Types of Immunostimulating Nanoparticles
- Dendrimers
Dendrimers are highly branched, synthetic nanoparticles with a well-defined structure. They have the ability to co-deliver antigens and adjuvants, such as CpG-ODN, a synthetic oligonucleotide that activates toll-like receptor 9. This co-delivery enhances dendritic cell maturation and antigen presentation, leading to a more robust T-cell response. Studies have shown that dendrimer-based vaccines can induce both innate and adaptive immune responses, making them promising candidates for cancer immunotherapy.
- Liposomes
Liposomes can be designed with pH-sensitive properties, which allow them to release their payload in the acidic environment of the tumor microenvironment or endosomes of APCs. This efficient antigen release ensures that the antigens are available for processing and presentation, maximizing the immune response. Liposomal formulations have been widely studied for their ability to deliver a variety of therapeutic agents, including peptides, proteins, and nucleic acids, in cancer vaccines.
- Iron Oxide Nanoparticles
Iron oxide nanoparticles have a dual role in cancer treatment. In addition to their use in magnetic resonance imaging for cancer diagnostics, they can also activate macrophages and T cells. By interacting with immune cells, they can modulate the immune microenvironment, promoting an antitumor immune response. Furthermore, their magnetic properties allow for targeted delivery using external magnetic fields, enhancing their specificity for tumor sites.
- Polymeric NPs (PLGA, PEG)
Polymeric nanoparticles, such as those made from poly(lactic-co-glycolic acid) (PLGA) and polyethylene glycol (PEG), are biodegradable and biocompatible, making them safe for clinical use. They can serve as carriers for both antigens and adjuvants, with the ability to control the release rate of the encapsulated agents. PEGylation of nanoparticles can also improve their circulation time in the body, reducing clearance by the reticuloendothelial system and enhancing their accumulation in tumor sites.
- Virus-Like Particles (VLPs)
VLPs are non-infectious particles that mimic the structure of viruses but lack the viral genetic material. They are highly immunogenic, as their repetitive structure resembles that of real viruses, which triggers a strong immune response. In cancer vaccines, VLPs can be engineered to display tumor antigens on their surface, effectively presenting these antigens to the immune system and inducing a robust antibody and T-cell response.
- Carbon Nanotubes & Nanogels
Carbon nanotubes are cylindrical structures with unique physical and chemical properties. They can be functionalized to target APCs, delivering antigens and adjuvants directly to these cells. Nanogels, on the other hand, are three-dimensional network structures that can absorb and retain large amounts of water, making them suitable for delivering hydrophilic therapeutic agents. Both carbon nanotubes and nanogels can modulate immunosuppressive cells in the tumor microenvironment, reversing the immune suppressive state and enhancing the efficacy of cancer vaccines.
Nanoparticles show promise in treating digestive tract cancers. In colorectal cancer, nanoliposome-RNA vaccines and polydopamine nanoparticles enhance anti-tumor responses. For gastric cancer, MOF nanoparticles combine photodynamic and immune therapies. However, clinical translation faces dosage, manufacturing, and regulatory hurdles. Future strategies include combination therapies, multi-antigen delivery, and AI-driven design. With continued research, nanotechnology could revolutionize cancer treatment, making it more personalized and effective.
Services you may interested in:
Controlled/Sustained-Release Platforms
- Controlled Release Vaccine Development Services: Converts multi-dose vaccines to single-dose via sustained-release tech, reducing injections and enhancing long-term immunity through gradual antigen release (e.g., polymer microspheres).
- ProSphere™ Printing Platform: High-speed printing produces monodisperse microspheres for linear release up to 6 months, minimizing pain and needle size.
Nanoparticle-Based Systems
- Nanoparticle Delivery Services: Designs carriers (liposomes, polymers) to boost antigen stability, immunogenicity, and targeted delivery via tailored particle properties.
- Liposome Development: Biodegradable lipid bilayers encapsulate antigens/adjuvants for versatile formulation and immune stimulation.
- Micelle Delivery: Self-assembling micelles (≤100 nm) target lymph nodes, enhancing dendritic cell activation and balanced immune responses.
- ISCOM-Based Systems: Cage-like nanoparticles induce robust Th1/Th2 and CTL responses, supporting mucosal administration.
Nano-Technologies for Formulation
- Nano Inclusion Technology: Solves poor solubility via hydrophilic-hydrophobic complexes for liquid delivery (e.g., tumor infusion), reducing dosage.
- NanoReactor Technology: Continuous precipitation synthesizes uniform micro/nanoparticles (5 nm–50 μm) for scalable production.
Nucleic Acid Delivery
- Linads™ Lipid Systems: pH-responsive lipid nanoparticles protect and deliver DNA/RNA, enabling efficient transfection and scalable formulation for nucleic acid vaccines.
General Optimization
- Delivery System Optimization: Holistic design improves efficacy, safety, and manufacturability across vaccine types via material science and immune profiling.
Each service is backed by Creative Biolabs’ expertise in preclinical development, ensuring cost-effective, high-quality solutions for global biopharmaceutical partners.
Reference
- Zdrehus, Razvan, Cristian Delcea, and Lucian Mocan. “Role of biofunctionalized nanoparticles in digestive cancer vaccine development.” Pharmaceutics3 (2024): 410. https://doi.org/10.3390/pharmaceutics16030410
