Creative Biolabs has launched a series of Next-IO™ programs to bring new medicines to the world. We are dedicated to being the trusted partner of drug discovery, development, and manufacturing. By building strong, productive relationships with our partners, we are confident to achieve their scientific and business goals that exceed expectations. This program aims to develop monoclonal therapeutic antibody (mAb) targeting amyloid-β that plays a critical role in Alzheimer's Disease.
Alzheimer's Disease
As a chronic neurodegenerative disease, Alzheimer's disease (AD) is considered as one of the most causes of dementia and elderly disability in old age. It can't be curable so far. Several hypotheses have been identified as the cause of AD, and in recent years, researchers have focused on the immunotherapies based on the amyloid-β hypothesis of AD. The accumulation of amyloid-β is considered as the early event in the AD process.
Amyloid-β
The amyloid-β (Aβ) is produced by amyloid precursor protein (APP) via a series of processes. As a transmembrane protein, APP is cleaved by α-secretase in normal conditions. When APP is cleaved by β-secretase 1 (BACE1), two components will be generated including sAPPβ and C99. C99 is cleaved by γ-secretase releasing Aβ which is believed to be one of the causes of AD. Aβ exists in various lengths, including Aβ1-40 and Aβ1-42. Aβ can aggregate to form oligomers, protofibrils, fibrils, and finally plaques which are one of the main features of AD pathology.
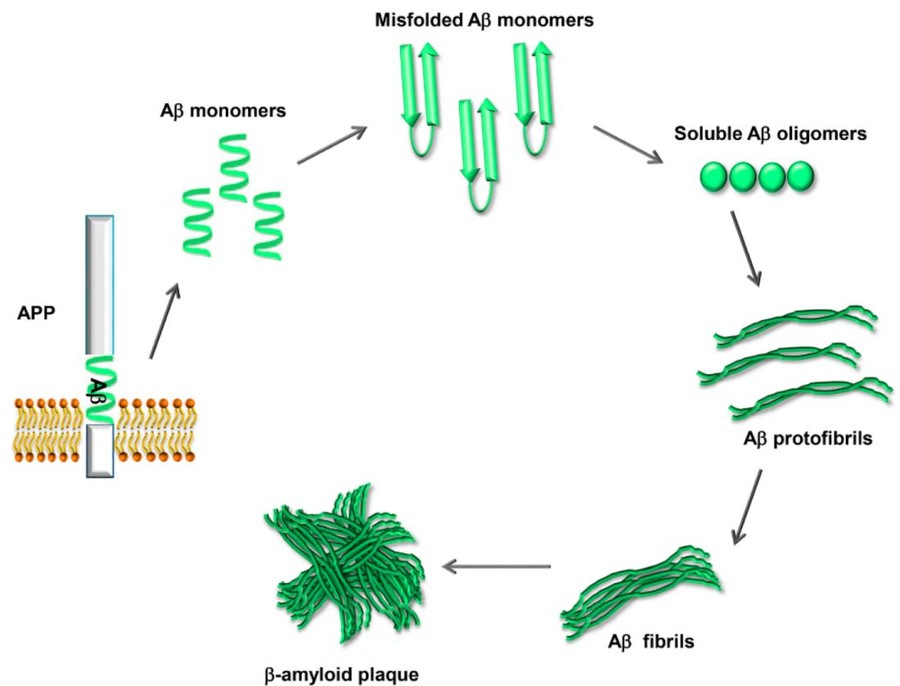 Fig.1 Human APP proteolytic pathway to generate Aβ.1
Fig.1 Human APP proteolytic pathway to generate Aβ.1
Anti-Amyloid-β Monoclonal Antibody Program
Many anti-Aβ drugs are currently under investigation or clinical trials to explore the efficacy of AD treatment targeting a wide range of targets (see Fig.3). While no one treatment has been approved so far. In Creative Biolabs, our scientists have extensive experience and expertise in drug discovery and development to treat AD. Furthermore, we have more than 10 years' experience in immunotherapy and antibody discovery and development. Empowered by our advanced and proprietary platforms, we are confident in the discovery and development of anti-Aβ monoclonal therapeutic antibodies.
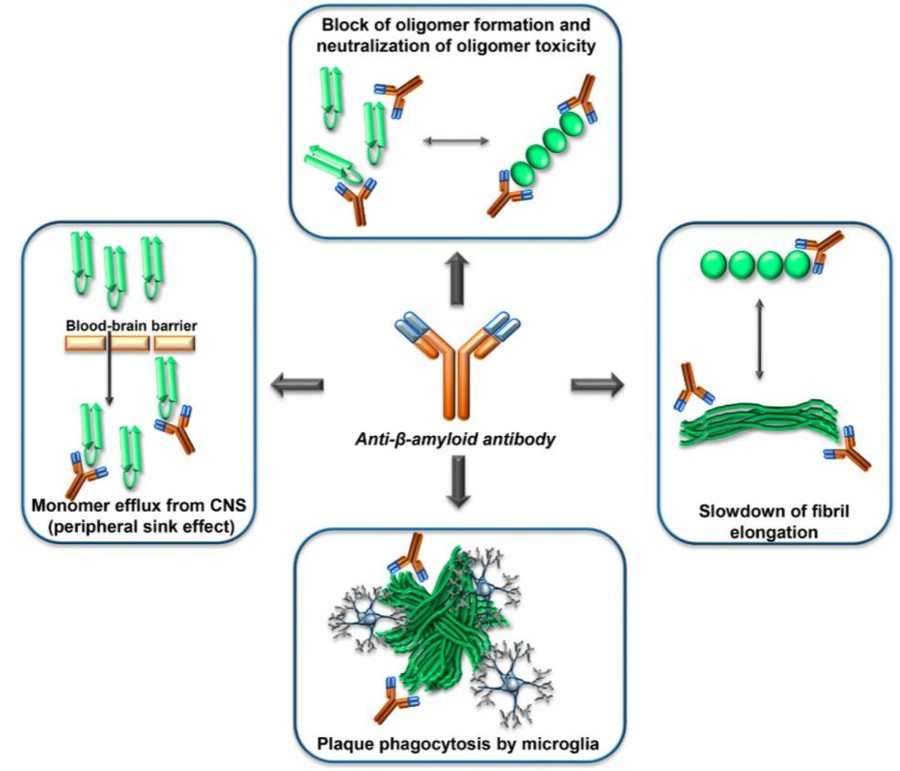 Fig.2 MOA of anti-Aβ-antibodies.1
Fig.2 MOA of anti-Aβ-antibodies.1
Market Prospect
Here are some listed drugs to treat AD. The trials of drugs marked in red have been discontinued or are inactive. Other drugs marked in blue are still under stages of clinical development. This program aims to develop the therapeutic mAbs targeting Aβ in different forms including oligomers, protofibrils, fibrils. For other targets, please visit our other programs in the AD area, or reach out to our scientists for assistance. We are confident in the future of promising targeting Aβ therapeutics.
With extensive experience in antibody discovery and development, Creative Biolabs is confident in our program promoting and management. For our Next-IO™ programs, we are committed to completing the pre-IND stage within about 2 years. The accurate timeline will be determined on a case-by-case basis. Here is a draft timeline for your glance.
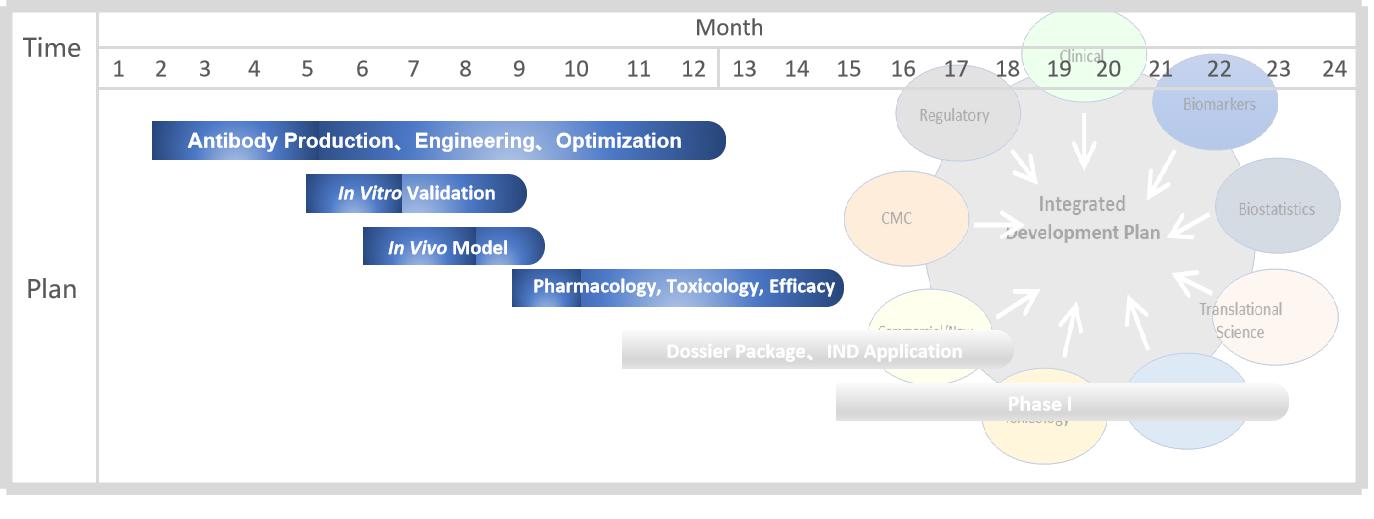 Fig.6 A brief program timeline.
Fig.6 A brief program timeline.
Creative Biolabs is seeking partners to co-develop the therapeutic monoclonal antibody program targeting Aβ. We are dedicated to providing our customers 24/7 high-standard customized service. Our mission is to discover and develop innovative medicines to facilitate your drug programs. We embrace partnerships, and if you are interested in our program, please feel free to contact us for further communication or TC meeting.



 Fig.1 Human APP proteolytic pathway to generate Aβ.1
Fig.1 Human APP proteolytic pathway to generate Aβ.1
 Fig.2 MOA of anti-Aβ-antibodies.1
Fig.2 MOA of anti-Aβ-antibodies.1

 Fig.6 A brief program timeline.
Fig.6 A brief program timeline.

 Download our brochure
Download our brochure