Diagnosis of Genetic Disorders
Genetic disorders result from abnormalities in the genome, the complete set of genetic information in an organism. These abnormalities can impact an organism's development, physiology, and metabolism, leading to various symptoms and complications. Genetic disorders, exceeding 6,000 known types, can either be inherited or acquired. Diagnosing these disorders is crucial for appropriate treatment and management, although it remains challenging due to their complexity, diversity, and rarity. Different techniques and methods have been developed to detect genetic abnormalities at various levels, such as chromosomes, genes, and proteins. These techniques have distinct advantages and limitations in terms of accuracy, sensitivity, specificity, cost, and availability.
Diagnosis of Genetic Disorders Associated with Chromosomal Abnormalities
Chromosomal abnormalities involve changes in the number or structure of chromosomes, the structures holding genetic information in cells. These abnormalities can cause disorders like Down syndrome, Klinefelter syndrome, Turner syndrome, and Fragile X syndrome, affecting an organism's development, physiology, and metabolism.
Chromosomal abnormalities are classified into two main types: numerical and structural. Numerical abnormalities occur when cells have abnormal chromosome numbers, such as aneuploidy (extra or missing chromosomes) or polyploidy (more than two sets of chromosomes). Structural abnormalities involve changes in a chromosome's shape or arrangement, such as translocation (parts of chromosomes attached to others), deletion (missing chromosome parts), duplication (extra copies of parts of chromosomes), inversion (reversed chromosome parts), or ring formation (chromosome ends joined together).
Cytogenetic techniques, including karyotyping, fluorescence in situ hybridization (FISH), and comparative genomic hybridization (CGH), are used to diagnose chromosomal abnormalities. Karyotyping identifies large-scale abnormalities, while FISH detects smaller-scale abnormalities like microdeletions or microduplications. CGH measures DNA amounts in each chromosome, identifying gains or losses of chromosomal material.
While cytogenetic techniques provide a comprehensive overview of chromosomal status and detect both numerical and structural abnormalities, they require sufficient high-quality cells. They may not identify subtle abnormalities that don't affect overall chromosome appearance or provide information on gene function or expression affected by chromosomal abnormalities.
Diagnosis of Rare Genetic Disorders
Rare genetic disorders, affecting a small number of people, result from mutations or DNA defects, causing diverse symptoms and complications in various organs and body systems. Examples include cystic fibrosis, sickle cell disease, Tay-Sachs disease, Huntington's disease, and Duchenne muscular dystrophy, classified into monogenic, mitochondrial, and complex disorders.
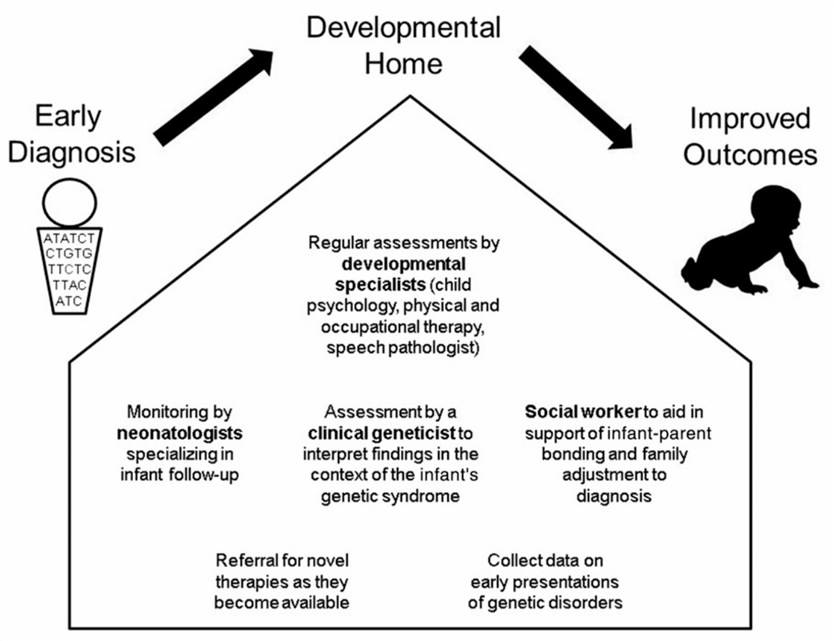 Fig.1 A developmental home for infants with rare genetic disorders. (Wojcik MH, 2020)
Fig.1 A developmental home for infants with rare genetic disorders. (Wojcik MH, 2020)
Monogenic disorders are caused by mutations in single genes that affect the function or expression of a protein. These mutations can be inherited in different patterns, such as autosomal dominant, autosomal recessive, X-linked, or Y-linked. Examples of monogenic disorders include cystic fibrosis, sickle cell disease, Tay-Sachs disease, and Huntington's disease.
Mitochondrial disorders are caused by defects in mitochondrial DNA or the nuclear genes that encode mitochondrial proteins. These defects affect energy production and cell metabolism. Mitochondrial disorders can be inherited from the mother (maternal inheritance) or from both parents (biparental inheritance). Examples of mitochondrial disorders include Leber's hereditary optic neuropathy, Kearns-Sayre syndrome, and Leigh syndrome.
Complex disorders arise from interactions between multiple genes and environmental factors. These interactions result in a multifactorial inheritance pattern influenced by the frequency and effect of each gene variant and environmental factor. Examples of complex disorders include autism spectrum disorder, schizophrenia, and type 2 diabetes.
To diagnose rare genetic disorders, molecular techniques are utilized. These techniques analyze DNA or RNA sequences or the protein products of genes using methods such as polymerase chain reaction (PCR), electrophoresis, high-performance liquid chromatography (HPLC), and DNA sequencing. These methods provide precise and accurate information about the molecular cause of a disorder, detect small or subtle abnormalities not visible by cytogenetic techniques, and offer insights into the function or expression of genes affected by mutations or variations. However, molecular techniques have limitations, such as requiring specialized equipment and expertise, not detecting complex or multifactorial disorders involving interactions between multiple genes and environmental factors, and not providing information about the clinical presentation or prognosis of a disorder.
Conclusion
In summary, genetic disorders arise from chromosomal changes or DNA mutations. This review has delineated the types and characteristics of these disorders, explaining their detection through cytogenetic and molecular techniques. It has assessed the advantages and limitations of these methods, highlighting current gaps and proposing suggestions for future research and development in this field.
References
- Lalonde E, et al. Genomic Diagnosis for Pediatric Disorders: Revolution and Evolution. Front Pediatr. 2020 Jul 8;8:373.
- Marwaha S, et al. A guide for the diagnosis of rare and undiagnosed disease: beyond the exome. Genome Med. 2022 Feb 28;14(1):23.
- Böhm S, et al. Children with a rare congenital genetic disorder: a systematic review of parents’ experiences and needs. Orphanet J Rare Dis. 2022 Feb 16;17(1):67.
- Boycott KM, et al. International Cooperation to Enable the Diagnosis of All Rare Genetic Diseases. Am J Hum Genet. 2017 May 4;100(5):695-705.
- Posey JE, et al. Resolution of disease phenotypes resulting from multilocus genomic variation. N Engl J Med. 2017 Jan 5;376(1):21-31.
- Wright CF, et al. Making new genetic diagnoses with old data: iterative reanalysis and reporting from genome-wide data in 1,133 families with developmental disorders. Genet Med. 2019 Jan;21(1):126-134.
- Vissers LE, et al. A clinical utility study of exome sequencing versus conventional genetic testing in pediatric neurology. Genet Med. 2017 Sep;19(9):1055-1063.
- Retterer K, et al. Clinical application of whole-exome sequencing across clinical indications. Genet Med. 2016 Jul;18(7):696-704.
- Stark Z, et al. A prospective evaluation of whole-exome sequencing as a first-tier molecular test in infants with suspected monogenic disorders. Genet Med. 2016 Nov;18(11):1090-1096.
- Farwell KD, et al. Enhanced utility of family-centered diagnostic exome sequencing with inheritance model-based analysis: results from 500 unselected families with undiagnosed genetic conditions. Genet Med. 2015 Jul;17(7):578-86.
- Iglesias A, et al. The usefulness of whole-exome sequencing in routine clinical practice. Genet Med. 2014 Dec;16(12):922-31.
- Wojcik MH, et al. Developmental Support for Infants With Genetic Disorders. Pediatrics. 2020 May;145(5):e20190629.
