Applications of Gene Therapy
Gene therapy presents a promising approach for treating various human diseases by introducing genetic material into the patient's cells. This innovative method can replace or correct defective genes, enhance or suppress gene expression, and modify the function or fate of cells. It employs diverse delivery methods, including viral and non-viral vectors, in vivo and ex vivo techniques, and gene editing technologies. However, gene therapy encounters numerous challenges, such as safety, efficiency, specificity, immunogenicity, and ethical concerns.
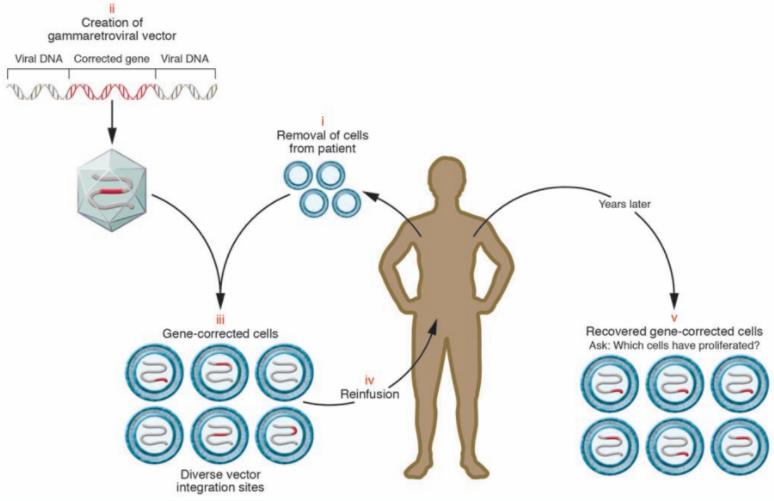 Fig.1 Gene Correction Using Retroviral Vectors (Bushman FD, 2007)
Fig.1 Gene Correction Using Retroviral Vectors (Bushman FD, 2007)
Prevention of Irradiation Damage to Salivary Glands
Salivary gland dysfunction frequently arises as a serious complication of irradiation therapy for head and neck cancer. Irradiation damages salivary gland cells, reducing saliva secretion and causing issues like xerostomia (dry mouth), dysphagia (swallowing difficulties), oral infections, dental caries, and diminished quality of life. Current treatments, such as artificial saliva, saliva stimulants, and anti-inflammatory drugs, are primarily palliative and symptomatic. However, these treatments often prove inadequate and may lead to side effects. Therefore, there is a pressing need for more effective and preventive therapies capable of protecting or restoring salivary gland function post-irradiation. Gene therapy emerges as a potential strategy, delivering genes that enhance the survival, proliferation, differentiation, or regeneration of salivary gland cells. Additionally, gene therapy can modulate the inflammatory response and the immune system, reducing salivary gland damage and fibrosis.
Autoimmune Disorders
Autoimmune disorders encompass a group of diseases where the immune system attacks the body’s own tissues and organs. These disorders affect various body parts, including joints, skin, nerves, blood vessels, and glands. Conditions like rheumatoid arthritis, Sjögren’s syndrome, and type 1 diabetes fall under this category. Although the exact causes and mechanisms remain unclear, factors such as genetics, environment, and hormones are believed to be involved. Current treatments for autoimmune disorders primarily focus on suppressing the immune system and reducing inflammation. However, these treatments often lead to adverse effects such as infections, malignancies, and organ toxicity. Moreover, these treatments are not curative and fail to address the root cause of autoimmunity. Hence, there is a pressing need for more specific and effective therapies capable of modulating the immune system and restoring immune tolerance. Gene therapy offers a promising avenue to achieve this objective by delivering genes that regulate the immune response and prevent or reverse tissue damage. This method can target various immune cells, including T cells, B cells, dendritic cells, and macrophages, and can also deliver antigens or cytokines to induce or suppress immune reactions.
Systemic Protein Deficiencies
Systemic protein deficiencies encompass a group of diseases arising from the absence or dysfunction of essential proteins crucial for the body's normal functioning. These deficiencies can impact various organs and systems, including the blood, lungs, liver, and muscles. Conditions like hemophilia, cystic fibrosis, and alpha-1 antitrypsin deficiency fall under this category. These deficiencies typically result from mutations or deletions in single genes responsible for encoding the deficient proteins. Current treatments, primarily centered around protein replacement therapy involving infusions of recombinant proteins or plasma-derived products, are expensive, inconvenient, and pose risks like immunogenicity, infection, and bleeding. Furthermore, these treatments are not curative and fail to address the underlying genetic defects. Thus, there's a pressing need for more permanent and specific therapies that can replace or correct defective genes or proteins. Gene therapy emerges as a potential solution, delivering functional copies of genes or proteins into the patient's cells.
Spinal Disorders
Spinal disorders comprise a range of conditions affecting the spinal cord's structure and its surrounding tissues, leading to symptoms such as pain, numbness, weakness, paralysis, and loss of bladder and bowel control. Examples include spinal cord injuries, intervertebral disc degeneration, and spinal muscular atrophy. The spinal cord, with its complex anatomy and delicate physiology, consists of millions of neurons and glial cells processing sensory and motor signals. Current treatments, primarily involving surgery, medication, and rehabilitation, are hampered by the spinal cord's limited regenerative capacity and irreversible neural tissue damage. These treatments do not fully restore the spinal cord's original function and sensation. Innovative and regenerative therapies are crucial to promoting spinal cord regeneration and repair. Gene therapy emerges as a promising approach, delivering genes capable of enhancing the survival, proliferation, differentiation, or regeneration of spinal cord cells. Additionally, gene therapy can modulate the inflammatory response and glial scar formation, hindrances to spinal cord recovery.
Gene Therapy for Cancer
Cancer is a group of diseases characterized by the uncontrolled growth and spread of abnormal cells that can invade and destroy normal tissues and organs. Cancer can arise from various genetic mutations and environmental factors that impact the regulation of the cell cycle, apoptosis, differentiation, and angiogenesis. Cancer cells exhibit several hallmarks, including self-sufficiency in growth signals, insensitivity to anti-growth signals, evasion of apoptosis, limitless replicative potential, sustained angiogenesis, and the ability to invade and metastasize in tissues. Cancer cells are also dynamic and heterogeneous, acquiring new mutations and adapting to different microenvironments. Current cancer treatments mainly involve surgery, chemotherapy, radiotherapy, and immunotherapy. However, these treatments often lack specificity, efficacy, and safety. Additionally, they do not provide a complete cure and fail to eradicate all cancer cells. Consequently, there is a demand for more targeted and effective therapies capable of selectively eliminating cancer cells and enhancing anti-tumor immunity. Gene therapy presents a potential strategy to achieve this goal by delivering genes that can modulate the expression or function of genes or proteins involved in cancer development or progression.
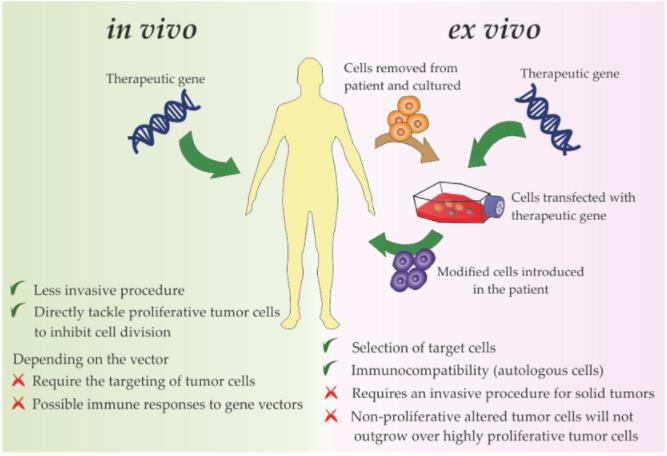 Fig.2 Delivery Strategies for Gene Therapy Directly Targeting Tumor Cells or Tumor Microenvironment Components (Roma-Rodrigues C, 2020)
Fig.2 Delivery Strategies for Gene Therapy Directly Targeting Tumor Cells or Tumor Microenvironment Components (Roma-Rodrigues C, 2020)
Gene Therapy for Eye Diseases
Eye diseases encompass a group of conditions that affect the structure and function of the eye and its components, including the retina, cornea, lens, and optic nerve. These diseases manifest with symptoms such as blurred vision, loss of vision, pain, inflammation, and infection. Examples of eye diseases include retinitis pigmentosa, age-related macular degeneration, glaucoma, and corneal dystrophy. The anatomy and physiology of the eye are intricate and sophisticated, comprising multiple layers and cells responsible for perceiving and processing light signals. Current treatments for eye diseases mainly involve surgery, medication, and devices. However, these treatments are often constrained by resource availability, accessibility, and affordability. Furthermore, they do not provide a complete cure and fail to fully restore the eye's original vision and function. Therefore, there's a need for innovative and regenerative therapies capable of preserving or restoring vision by targeting various aspects of the eye. Gene therapy emerges as a promising approach to achieve this goal by delivering genes that can modulate the expression or function of genes or proteins involved in the development or progression of eye diseases.
Gene Therapy for Cardiovascular Disorders
Cardiovascular disorders constitute a group of diseases affecting the structure and function of the heart and blood vessels, leading to symptoms like chest pain, shortness of breath, palpitations, edema, and stroke. Examples of cardiovascular disorders include coronary artery disease, heart failure, arrhythmia, hypertension, atherosclerosis, and peripheral vascular disease. The cardiovascular system's anatomy and physiology are intricate and vital, comprising multiple chambers, valves, and vessels responsible for blood and oxygen circulation throughout the body. Current treatments for cardiovascular disorders mainly involve surgery, medication, and devices, but these treatments are often limited by resource availability, accessibility, and affordability. Moreover, they do not provide a complete cure and do not address the underlying causes of cardiovascular disorders. Therefore, there's a need for more specific and effective therapies capable of improving cardiac function and vascular health by targeting various mechanisms. Gene therapy represents a potential strategy to achieve this goal by delivering genes that can modulate the expression or function of genes or proteins involved in the development or progression of cardiovascular disorders.
References
- Friedmann T, Roblin R. Gene therapy for human genetic disease? Science. 1972 Mar 10;175(4025):949-55.
- Bushman FD. Retroviral integration and human gene therapy. J Clin Invest. 2007 Aug;117(8):2083-6.
- Stamatoyannopoulos G, Nienhuis AW. Hemoglobin switching and the new era of gene therapy for hemoglobinopathies. Blood. 2019 Oct 31;134(18):1445-6.
- Naldini L, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996 Apr 12;272(5259):263-7.
- Papanikolaou E, Bosio A. The Promise and the Hope of Gene Therapy. Front Genom Ed. 2021 Mar 24;3:618346.
- Uddin F, Rudin CM, Sen T. CRISPR Gene Therapy: Applications, Limitations, and Implications for the Future. Front Oncol. 2020 Aug 7;10:1387.
- Baum C, et al. Side effects of retroviral gene transfer into hematopoietic stem cells. Blood. 2003 Feb 15;101(4):2099-114.
- Cavazzana M, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000 Apr 28;288(5466):669-72.
- Hacein-Bey-Abina S, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003 Oct 17;302(5644):415-9.
- Aiuti A, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009 Jan 29;360(5):447-58.
- Roma-Rodrigues C, et al. Gene Therapy in Cancer Treatment: Why Go Nano? Pharmaceutics. 2020 Mar 5;12(3):233.
- Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014 Oct 16;371(16):1507-17.
- Maguire AM, et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009 Nov 7;374(9701):1597-605.
- Bainbridge JW, et al. Long-term effect of gene therapy on Leber’s congenital amaurosis. N Engl J Med. 2015 May 14;372(20):1887-97.
- Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014 Nov 28;346(6213):1258096.
- Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013 Feb 15;339(6121):819-23.
- Maeder ML, Gersbach CA. Genome-editing technologies for gene and cell therapy. Mol Ther. 2016 Mar;24(3):430-46.
- Frangoul H, et al. CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. N Engl J Med. 2021 Jan 21;384(3):252-60.
- Stadtmauer EA, et al. CRISPR-engineered T cells in patients with refractory cancer. Science. 2020 Feb 28;367(6481):eaba7365.
