Protein drugs have high risks of inducing immunogenicity, which may affect the efficacy or even be life-threatening. Most biotherapies, such as antibody-drug conjugates (ADCs), fusion proteins, and polyethylene glycolization, carry unnatural human protein sequences or structural motifs that may increase the risk of immunogenicity. Comprehensive risk assessment strategies and analytical methods are required to monitor and characterize the immunogenicity of ADC and other biotherapies in order to understand and predict potential clinical effects.
The monoclonal antibody (mAb) in an ADC is covalently linked to the cytotoxic agent through a stable conjugate, which combines the specificity of the mAb to tumor cell surface target antigens and the high efficacy of cytotoxic drugs. Although current ADC uses humanized mAbs and small molecular payloads, its hapten structure may increase the risk of immunogenicity compared with therapeutic mAbs.
Anti-drug antibody (ADA) can target different domains of an ADC, such as mAb epitopes, novel mAb epitopes, connectors, and cytotoxic agents. If large ADC-ADA immune complexes are ingested by non-targeted immune cells, resulting in cell death, then anti-cytotoxic ADAs have a safety risk.
It is generally believed that the practices and regulatory guidelines of the industry used to evaluate the immunogenicity of biotherapeutic drugs can be applied to ADC. The key to the evaluation of ADC immunogenicity is risk assessment, appropriate detection, and additional characteristics of ADA domain specificity. Immunogenicity risk assessment covers a variety of known factors related to patients and products, which may affect the immunogenicity of drugs and the potential consequences of immune responses. The safety risk of ADC is generally considered higher than that of therapeutic monoclonal antibodies.
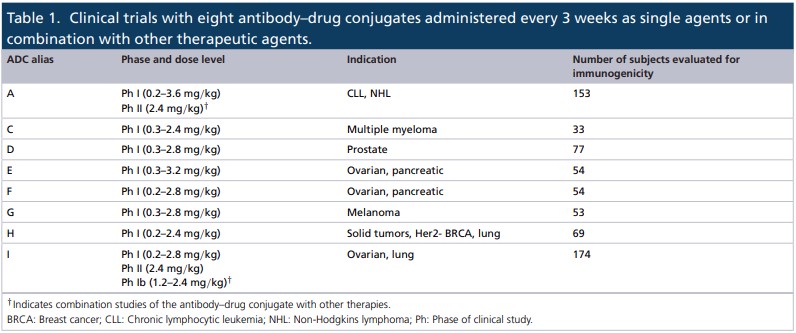
Based on risk assessment, the immunogenicity data of 8 ADC drugs were analyzed in 11 clinical trials involving a range of solid and hematological tumor indications. All ADCs’ incidences of ADA at baseline, the incidences after treatment, and other characteristics of immune response was evaluated.
-
Baseline incidence of ADAs
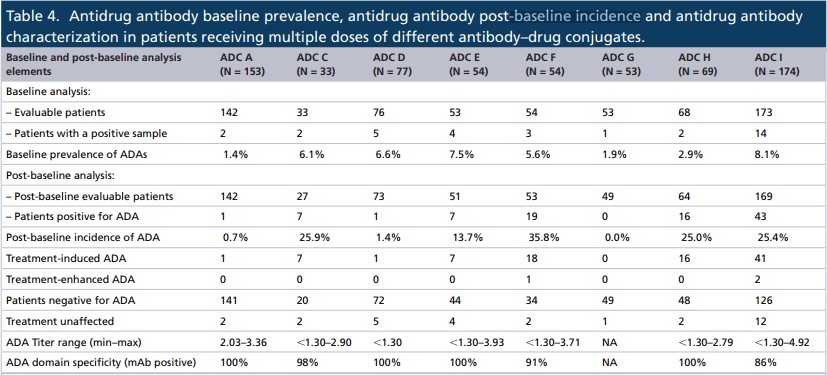
According to the research data, the baseline incidence of ADAs is between 1.4% and 8.1%, which is within the range reported by other monoclonal antibody-derived biotherapies, including the ADC drug—Kadcyla.
In the clinical studies of those 8 ADC antibodies, they are unlikely to be related to other components of ADC. In addition, of the 8 ADC evaluated, the background signal in the untreated patient population was similar to the mixed serum control signal from healthy volunteers.
2. Incidence of ADAs after ADC treatment
The incidence of ADAs after baseline was between 0 and 35.8%. There were fewer ADC patients targeting hematological tumors than ADA patients targeting solid tumors, which can be attributed to the immune killing of patients with hematological tumors.
Overall, those data indicate that the hapten structure of these ADCs is unlikely to increase the risk of immunogenicity in most patients compared with traditional therapeutic monoclonal antibodies.
As for the ADA level, the total titer of those ADCs ranged from less than 1.30 to 4.92, and the average titer of all ADCs exceeded 2.50.
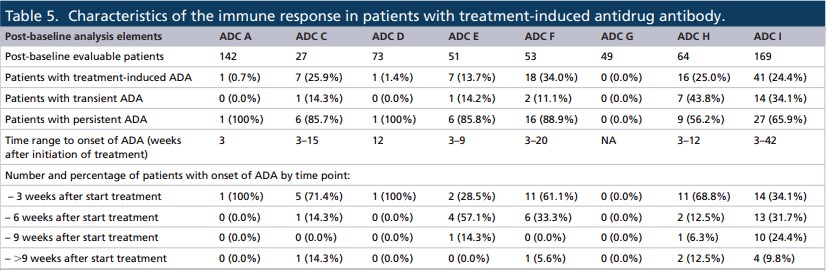
It showed that the time of occurrence of treatment-induced ADA was variable in all ADCs, usually between 3 and 42 weeks. In addition, 6 of the 8 ADCs developed ADAs in more than 60% of patients within 3 to 6 weeks after treatment.
Most of the responses of all ADCs were persistent, ADC H and I had the highest transient responses (43.8% and 34.1%, respectively). However, classifying the ADA response as persistent or transient in oncology studies may be misleading because the treatment time is usually short. In fact, a more detailed assessment of the ADA positive response data showed that the three ADCs (F, H, and I) had higher ADA positive rates.
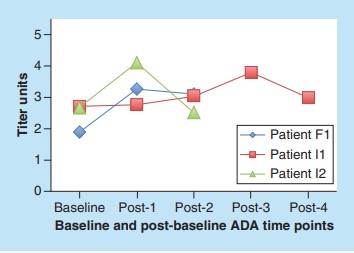
Of the 652 patients, 33 had antibodies at baseline, and 3 had increased ADA response. The ADA titers of the three patients varied over time, with baseline titers ranging from 1.89 to 2.71 and post-baseline titers from 2.50 to 4.10. 2 of the 3 patients had peak ADA at the time point after the first baseline after treatment, and the time range of enhanced response was 3 to 9 weeks. The level of ADA in all patients at the last time point was lower than the peak level. The domain specificity of ADA remained unchanged at baseline and post-baseline time points.
3. The impact of ADA
Immunogenicity is an important part of the clinical characteristics of protein drugs, of which the data should be evaluated in the context of other factors such as efficacy, competition, and safety. Here we focus on the three ADCs, F, H, and I, with a high number of patients producing ADA, with 19, 16, and 43 post-baseline ADA positive patients, respectively.
The incidence of ADAs in ADC F was 35.8% (19/53), and 18 patients were treatment-induced ADAs. 1 and other patients were treatment-enhanced ADAs. Overall, no differences were observed in terms of competition, safety, or efficacy outcomes compared with patients who did not produce ADC F antibodies. In addition, the titer of ADA has an effect on the competition of these patients, but it has been previously reported in cynomolgus monkeys.
As for ADC-H, the incidence of ADAs is 25% (16/64). All 16 patients had treatment-induced ADAs. Similar to ADC-F, the trough level of total antibody in some patients with higher ADA titer was lower than that in patients with lower ADA titer, but the patients with higher total ADA titer did not correspond to those with lower total antibody titer.
A total of 43 patients with ADC-I had ADAs, 41 induction, and 2 patients enhancement. It is worth noting that the total antibody levels of 7 patients (including 2 patients with ADAs enhancement) were lower than the detected levels at one or more trough points. However, a comprehensive analysis is still needed to conclude that ADA titers have an impact on the competition of these patients. However, it is worth noting that the high titer of ADA (~4.00) has a significant effect on the competition curve of trastuzumab ADC in cynomolgus monkeys.
Limited data obtained from listed ADCs show that ADAs has little impact on clinical outcomes. The incidence of ADAs of Adcetris was 37%, and the infusion response frequency of patients with persistent positive ADAs was higher, resulting in the withdrawal of treatment in two patients. The incidence of ADAs of Kadcyla is 5.3%. The development of Perry ADA seems to have no effect on safety, competition, or efficacy. In the phase I study, there were some ADA-positive patients treated with Mylotarg, including one with transient shortness of breath associated with ADAs. Under the currently recommended dose regimen, no additional immunogenicity data for Mylotarg was generated in clinical trials. In the clinical trial of Besponsa, the incidence of ADAs was 3%, which had no effect on the clearance rate of ADC.
Summary
The above experimental results show that the hapten-like structure plays a very small role in the immune response of ADC. These results reinforce the fact that molecular structure is an important but not the only factor in the immune response to biotherapy.
Although data from the eight ADCs indicate that the risk may be reduced as the project enters a later stage of clinical development, a conservative approach is still recommended for these new ADCs. In addition, as predictive immunogenicity tools become more common, they will provide additional information for inclusion in risk assessment and immunogenicity strategies for ADCs and new treatments.
References:
Immunogenicity of antibody-drug conjugates: observations across 8molecules in 11 clinical trials. Bioanalysis. 2019 Sep;11(17):1555-1568