Recently, Amgen announced the long-term efficacy of the company’s bispecific antibody Blincyto (blinatumomab) in the treatment of patients with acute lymphoblastic leukemia (ALL) at the European Hematology Association (EHA) conference. The median overall survival (OS) of patients receiving Blincyto was 36.5 months at a median follow-up of 59.8 months.
ALL is a rapidly progressing blood cancer. The patient can enter complete hematologic remission after receiving chemotherapy. However, some of these patients continue to develop the minimal residual disease (MDD). MRD means that although the patient has entered complete hematologic remission using routine testing, the presence of cancer cells can still be detected in the bone marrow using highly sensitive detection methods, and the current sensitivity of MRD detection is detecting one cancer cell in 10, 000 cells.
MRD is considered to be the most important factor in determining the prognosis of ALL patients, and MRD-positive patients usually have a poor prognosis.
Blincyto is a bispecific T-cell engager (BiTE) molecule that Amgen has developed to target both CD19 and CD3 based on its BiTE platform. One end can bind to the CD19 antigen on the surface of the B cell, and the other end can bind to the CD3 receptor on the surface of the effector T cell, thereby recruiting T cells to the vicinity of the B cell to attack them. It is the first FDA-approved bispecific antibody on the Amgen BiTE technology platform.
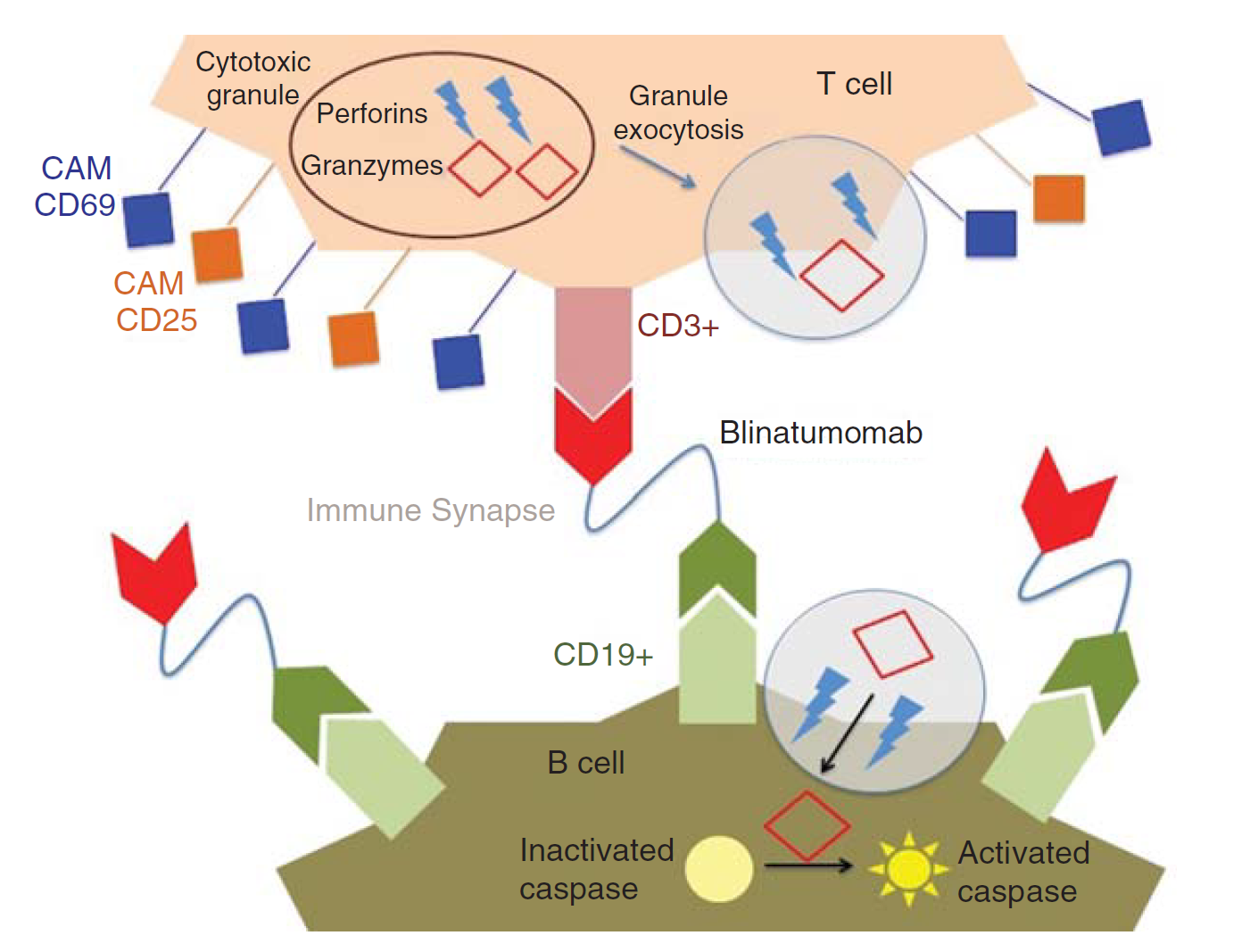
Fig.1 Mechanism of action of blinatumomab. (Rogala, 2015)
In a phase 2 clinical trial called BLAST, MRD-positive precursor B-cell ALL patients were treated with Blincyto. These patients have received more than 3 rounds of intense chemotherapy, and although complete hematologic remission, MRD continues to show positive. These patients can receive up to 4 rounds of Blincyto treatment and can choose to receive hematopoietic stem cell transplantation at any time after treatment.
The trial results showed that the median OS reached 36.5 months in 110 patients who underwent OS assessment. Of these, 84 patients had a negative MRD after receiving Blincyto, and the median OS of these patients had not yet arrived. MRD continued to be positive in 23 patients after treatment, and the median OS of these patients was 14.4 months.
“MRD-positive is an important predictor of disease recurrence in patients with precursor B-cell ALL,” Nicola G, one of the BLAST research leaders. Dr. Kbuget said: “The 5-year follow-up data from the BLAST trial showed that treatment with Blincyto allows patients to achieve MRD-negative complete hematologic remission as early as possible, prolonging patient survival.”
Reference
1. Smith, Eric J., et al. “A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys.” Scientific reports 5.1 (2015): 17943.
