In 1960, Nisonoff and his collaborators at the Roswell Park Memorial Institute in New York first proposed the original concept of bispecific antibodies (BsAbs). Since then, with the great progress in the field of antibody engineering and antibody biology, the concept and technology of constructing BsAbs have continued to innovate.
Up to now, there are more than 100 BsAbs construction models, including but not limited to Tandem scFv, Tandem Fab , Diabody, IgG-scFv, CrossMab, IgG-IgG, Triple Body, Fab-IgG, TandAb, Single-chain Diabody, Single-chain Triplebody, Fab-Fv, Minibody, Bispecific Immunotoxin, scFv-based Bispecific Fragments, Bibody, Constant Domain-extended Bispecific Fragments, Appended IgGs, Fab-scFv Fusion Proteins, Single chain IgGs.
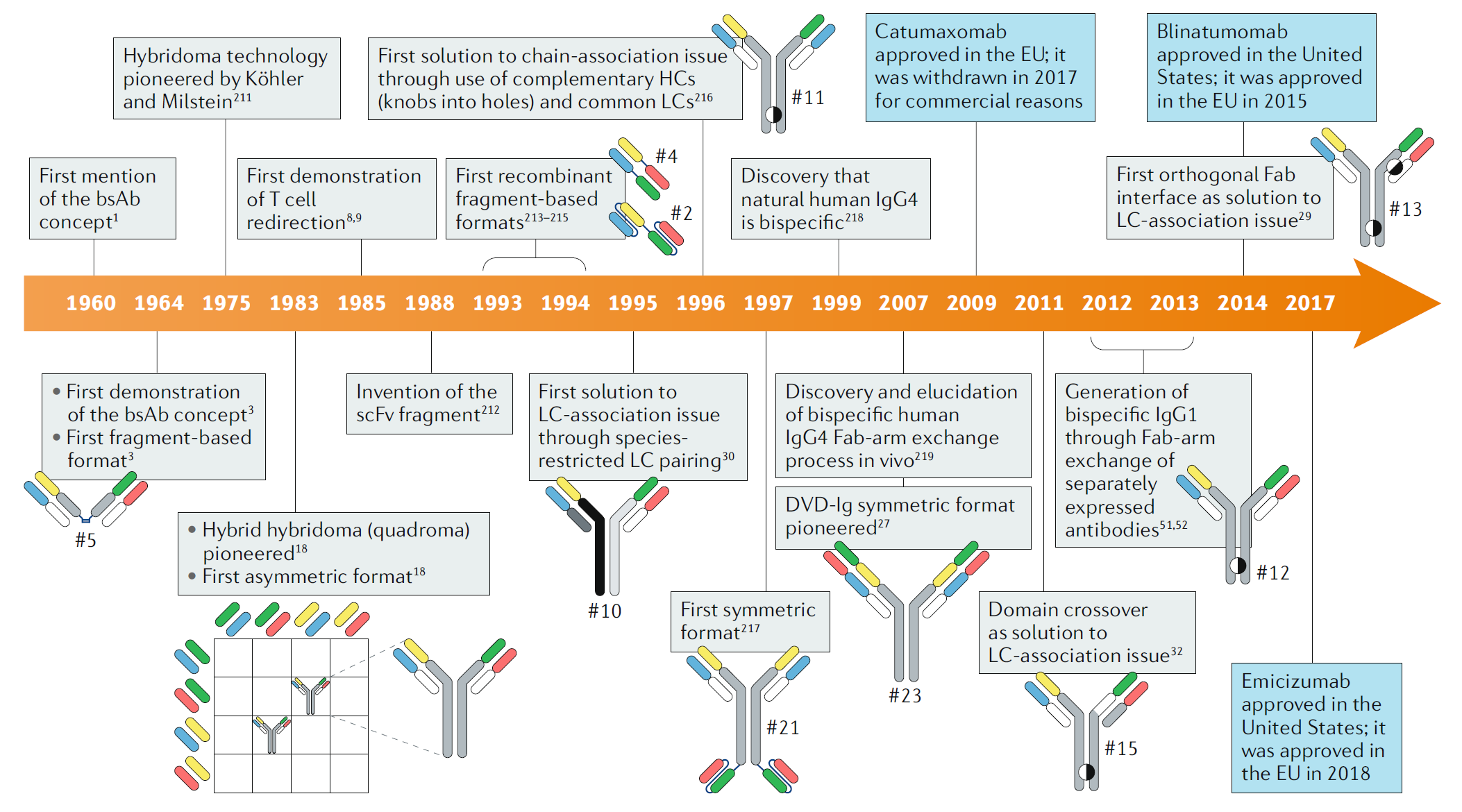
Fig. 1 Timeline of conceptual and technical innovations contributing to the development of the therapeutic bsAb landscape. (Labrijn, 2019)
Although BsAb has great clinical treatment prospects and commercial development value, their transformation into clinical therapies has proven to be challenging. Currently, there are only three types of double antibodies approved in the world:
| Drug Name | Target | Company | Time of Approval | Indication |
| Catumaxomab | EpCam/CD3 | Trion | 2009/4 (EMA) | Malignant ascites |
| Blinatumomab | CD3/CD19 | Amgen | 2014/1 (FDA) | Relapsed or refractory B-cell precursor acute lymphoblastic leukemia |
| Emicizumab | FIX/FX | Roche | 2017/11 (FDA) | Hemophilia A |
In 2009, the first BsAb, catumaxomab (targeting CD3 and EpCAM), was approved by the European Union for the treatment of malignant ascites. But this drug was withdrawn from the market in 2017 due to commercialization reasons. What is quite dramatic is that in the year of delisting, Horst Lindhofer, the inventor of this BsAb, founded a joint venture in China, and restarted catumaxomab’s listing application in 2019. At present, this drug has been in Phase I clinical trials in the treatment of gastric cancer and bladder cancer.
This new drug has been developed, marketed, delisted, and then re-applied for listing within the past two decades, which shows that the development of a new drug is not that easy, and it requires a lot of time and money as well as the faith and persistence of both scientists and entrepreneurs.
The main reason why the development of BsAb is difficult is that these antibodies do not exist in nature and need to be produced by artificial methods. There are three main methods: recombinant DNA method, cell hybridoma technology, and chemical coupling method. Among them, the recombinant DNA method is most commonly used, and there are three challenges:
(1) Structural Design
Although the market prospect is clear, the application-based antibody structure design is undoubtedly one of the difficulties in the development of BsAb. This requires that at the beginning of development, attention should be paid to how to balance and coordinate the safety, effectiveness, dosage and period of administration, and affinity with different epitopes of the two targets. Of course, patent issues must also be considered in advance. Specific structural adjustments also require clinical investigation, accumulation and verification in the later stage.
(2) Technology Platform
Currently, the dual-specific technology platforms that have been developed have their own characteristics. There are dozens of them, but continuous exploration and optimization are still required to develop platforms and technologies with production feasibility and scalability, as well as the versatility. The introduction of BsAb technology platforms with different characteristics from abroad is also one of the measures to be considered.
In the development and preparation of BsAb, the key is to have its own structure and preparation technology platform. In recent years, Roche, Johnson & Johnson, Amgen, Pfizer, Servier, Takeda and other multinational pharmaceutical companies have invested heavily in the development of BsAb. Taking Roche as an example, more than 30% of the macromolecular drug R&D pipeline is deployed in BsAb. In addition, on the basis of internally developing its own technology platform and expanding the R&D pipeline, Roche also actively introduces other different technology platforms to strengthen its technical reserves.
(3) Preparation and Industrialization
BsAb has a special structure, and it is difficult to realize the structural features required for its functions. Moreover, molecular stability, immunogenicity, and pharmacokinetic properties are changed due to structural adjustments, making molecular preparation and industrialization of BsAb much more difficult than that of monoclonal antibodies (mAbs). This is also an important factor that needs to be considered at the beginning of development.
In addition, if the effect of a BsAb drug is only equal to or a little more than the effect produced by the combination of two mAbs, it is not the most ideal one. The ideal BsAb drug can produce brand-new functions and effects, and this new function cannot be obtained by the combination of two mAbs. This puts forward higher clinical value requirements for BsAb drugs.
So far, since most of the BsAb products at home and abroad are still in the clinical stage I/II, there is still a long way for BsAb to shine in the market, especially in the industrialization. However, with decades of development of BsAbs, they are constantly maturing and making breakthroughs in terms of concepts and technologies, and they will surely have a profound impact on future disease treatments.
Reference
1. Labrijn, Aran F., et al. “Bispecific antibodies: a mechanistic review of the pipeline.” Nature Reviews Drug Discovery 18.8 (2019): 585-608.
