Amgen, the United States biotechnology giant, recently announced that the Ministry of Health, Labor and Welfare (MHLW) has approved bispecific T cell engager immunotherapy Blincyto® (blinatumomab) for the treatment of relapsed or refractory B-cell acute lymphoblastic leukemia (ALL) in children and adults. In Japan, Blincyto was developed by Amgen Astellas BioPharma K.K. (AABP), a joint venture between Amgen and Astellas.
About Blincyto
Blincyto is the first and only bispecific T cell-based immunotherapy approved worldwide. It is the first bispecific antibody product produced by Amgen bispecific T cell engager technology platform, which can present CD19 protein on tumor cells. The CD3 protein specifically expressed by T cells activates the immune system to recognize and kill tumor cells. To date, Blincyto has been approved by 57 countries, including the United States, the European Union, and all member states of the European Economic Area, Canada and Australia.
The approval is based on data from several global clinical studies, including the Phase III clinical study TOWER and the Japanese Ib/II clinical study Horai. In the TOWER study, Blincyto showed superiority in median overall survival compared with standard care (SOC) chemotherapy (median OS: 7.7 months [95% CI: 05.6-9.6] vs 4.0 months [95] %CI: 2.9-5.3], p=0.012). The Horai study was conducted in 35 adults and pediatric patients with relapsed or refractory B cell precursor ALL. Safety data from this study were consistent with other global clinical studies, including TOWER.
AABP President Steve Sugino said: “Blincyto’s approval marks an important milestone. B-cell ALL is one of the most aggressive B-cell malignancies, and we are proud that Blincyto was the company’s first cancer therapy approved in Japan. It will provide a much-needed innovative treatment option for children and adults with relapsed or refractory B-cell ALL.”
About bispecific T cell engager immunotherapy
Bispecific T cell Engager technology is an innovative investigational approach designed to help engage the body’s endogenous T cells to target malignant cells. Like other bispecific antibodies (bsAbs), and unlike ordinary monoclonal antibodies (mAbs), bispecific T cell engagers form a link between T cells and tumor cells, which makes T cells to exert cytotoxic activity on tumor cells by producing proteins like perforin and granzymes, without the presence of major histocompatibility complex class I (MHC-I) or co-stimulatory molecules. These proteins enter tumor cells and initiate the cell apoptosis, mimicing physiological processes observed during T cell attacks against tumor cells.
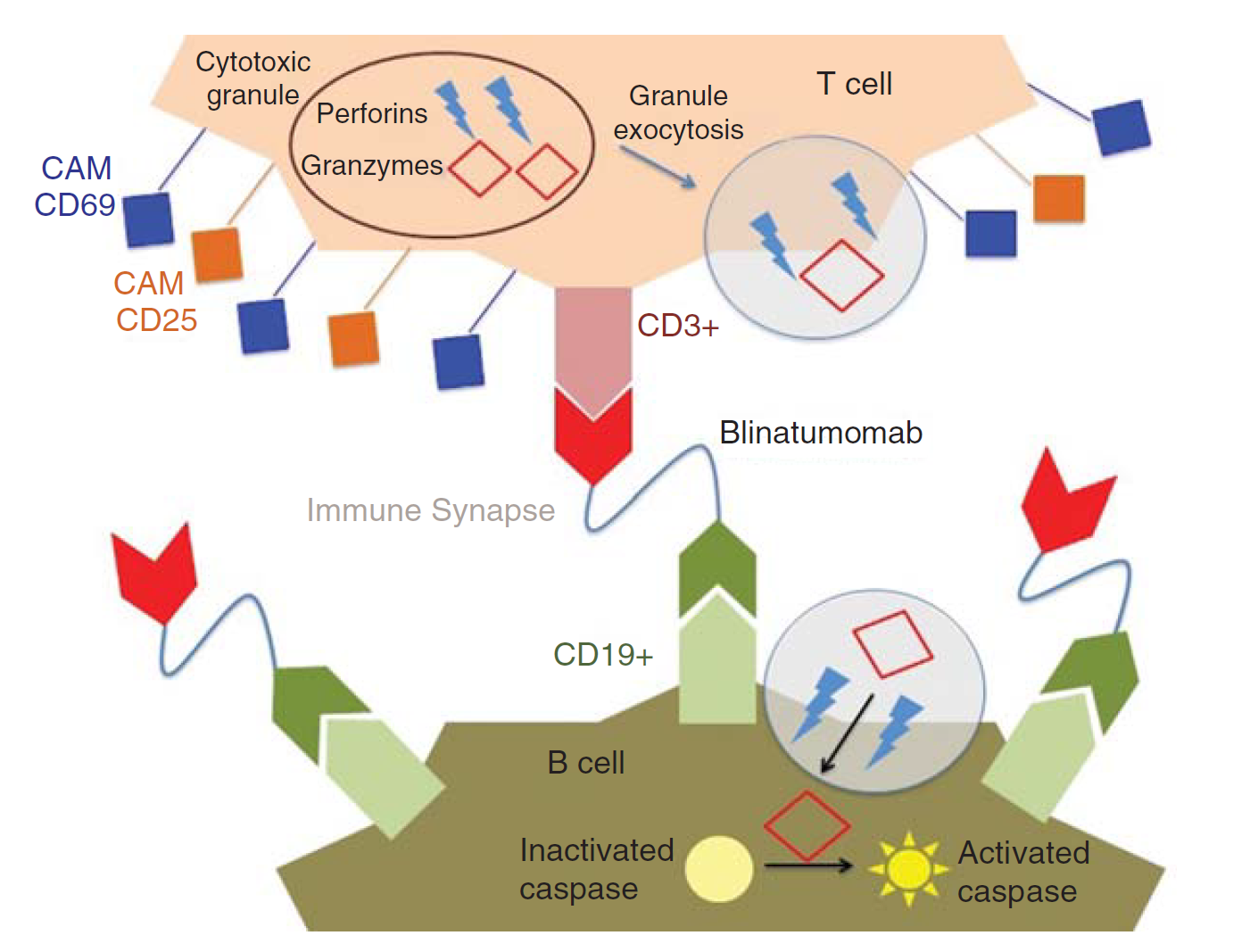
Fig.1 Mechanism of action of blinatumomab. (Rogala, 2015)
Amgen acquired bispecific T cell engager technology after acquiring the Micromet company for $1.2 billion in 2012. Currently, Amgen is exploring the potential of bispecific T cell engager’s innovative therapies in a wide range of refractory tumor types. Previously, both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have granted blinatumomab the status of orphan drugs and breakthrough therapy for the treatment of various types of hematological cancers, including acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), hairy cell leukemia (HCL), and young Lymphocytic leukemia (PLL) and inert B-cell lymphoma, mantle cell leukemia (MCL), and the like.
Reference
1. Rogala, Britny, et al. “Blinatumomab: enlisting serial killer T-cells in the war against hematologic malignancies.” Expert opinion on biological therapy 15.6 (2015): 895-908.
