Nowadays, in the tide of research and development of biological agents, antibody drugs are in an explosive period. The major monoclonal antibody (McAb) drugs have accounted for more than half of the global biological drug market for many years, especially in recent years, and the breakthrough of PD-1/PD-L1 immunosuppressants in the field of cancer treatment has further promoted its rapid development. On this basis, innovative strategies such as bispecific/multispecific antibodies and antibody drug conjugates (ADC drugs) have showed us the great potential for further development in this field. Compared with traditional McAbs, the multispecific antibody strategy with two or more targets has the advantage of more accurate targeting and stronger therapeutic effect, which fully stimulates the enthusiasm of research and development. However, due to the high R & D barriers, the number of approved drugs on the market is far less than that of monoclonal antibodies. Up to now, there are only two bispecific antibodies in the market, and there are few projects in phase III clinical trials.
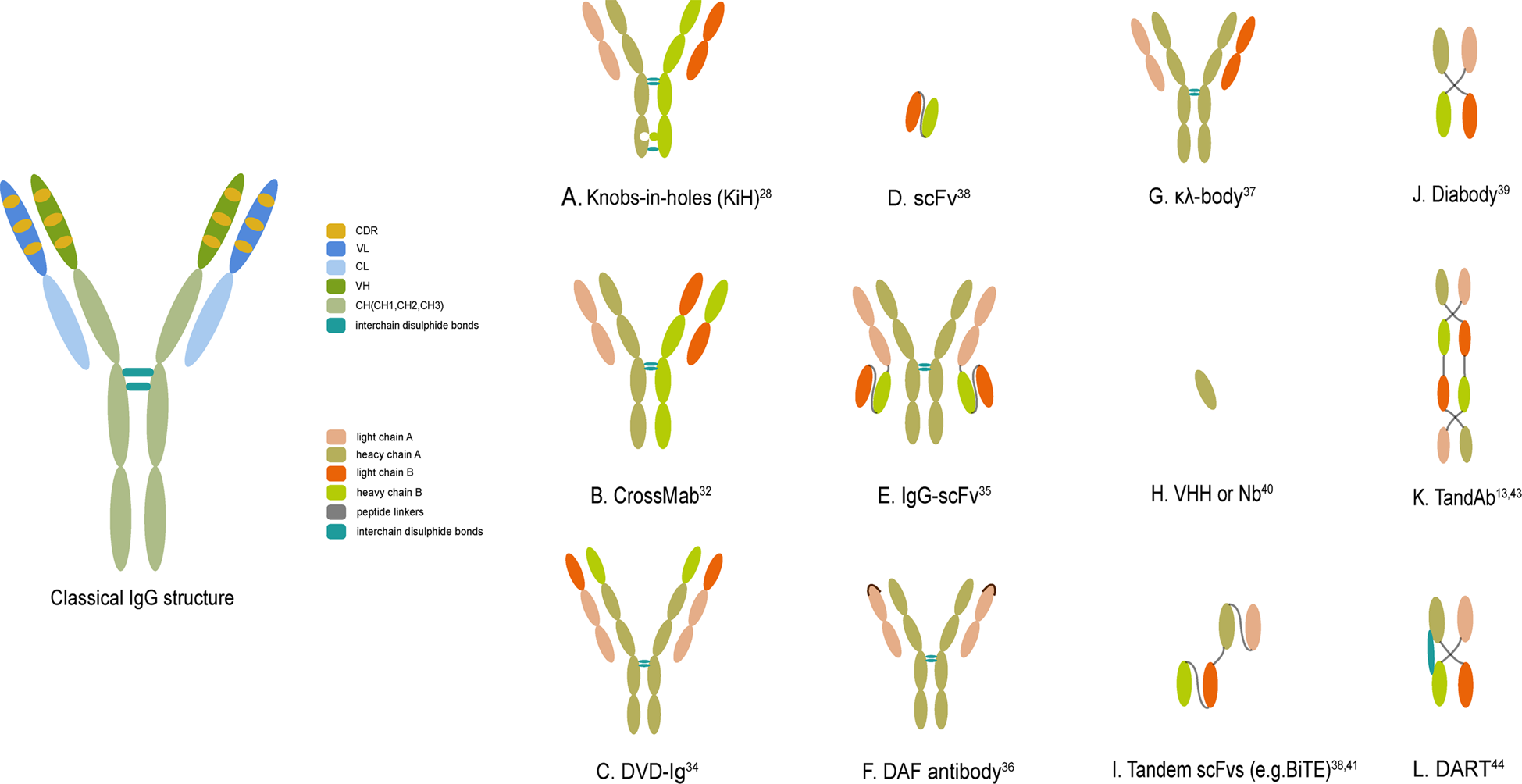
Fig. 1 The classical IgG structure and schematic representations of several crucial bsAb formats. (Li, 2020)
Bispecific antibody (BsAb) refers to an antibody molecule that targets two antigens or two different epitopes of one antigen at the same time. Compared with ordinary antibodies, bispecific antibodies add a specific antigen binding site, so compared with common antibodies, bispecific antibodies are more specific, more accurate in targeting tumor cells and reducing off-target toxicity. The combined application of antibodies based on bispecific antibodies targeting two disease antigens brings a good synergistic effect, which promotes people’s enthusiasm for this kind of antibodies.
Three big mountains of Bispecific Antibody research and development
In the more than ten years since the first bispecific antibody drugs were put on the market, only three bispecific antibody drugs have been approved to go on the market so far. Among them, the first bispecific antibody catumaxomab (targeting CD3 and EpCAM) used to treat malignant ascites was withdrawn from the market in 2017 for commercial reasons. Most of the others are in the stage of clinical research. The reason is that structural design, technical platform and evaluation model make it difficult to develop bispecific antibody.
- Structural design
The earliest bispecific antibodies combined two different heavy chain (H) and two different light chain (L) by chemical coupling or cell fusion. However, this random combination can produce 16 different combinations, from which it is difficult to separate the target combination. In the following decades, bispecific antibodies mainly focused on how to effectively combine two different antigen recognition sites while improving the uniformity and yield of target antibodies.
- Appropriate preclinical evaluation model
An appropriate preclinical evaluation model is also an unavoidable problem in the research and development of bispecific antibodies. At the beginning of development, it is necessary to balance and coordinate the security and effectiveness of the two targets. However, the conventional animal model does not have the same target binding characteristics as human, for example, the expression of the target is different from that of human, the binding ability and the pharmacological action are different, and the upstream and downstream signals of humanized animal model are also different. As a result, it is difficult for the preclinical evaluation model to correctly evaluate the rationality, pharmacological and toxicological effects of target design, which increases the difficulty of evaluating the rationality of target design.
Current preclinical evaluation models usually use alternative molecular, humanized animal and Minimal Anticipated Biological Effect Level (MABEL) methods. Although these models can reflect the action mechanism, pharmacological action, toxic target and toxic phenomenon of bispecific antibody to some extent, the effective dose, toxic dose and side effect obtained from preclinical evaluation due to species target expression, binding ability, pharmacological action, drug competition and species difference. Generally speaking, the conversion of clinical dose can not be carried out directly, and it may even lead to a certain degree of misleading and increase the risk of clinical research.
Therefore, it is necessary to develop more suitable preclinical evaluation models and methods of bispecific antibodies, or relax the experimental restrictions on high-grade primates, such as chimpanzees, so as to better carry out the preclinical evaluation.
- Technology platform
Another difficulty in the development of bispecific antibodies is the preparation of technology platforms. The bispecific technology platforms developed at the present stage have their own characteristics, and there are dozens of them at present, but they still need to be constantly explored and optimized to develop a platform technology with both medicinal properties, production process feasibility and scalability, at the same time, the versatility of the production platform should also be taken into account.
The prospect of bispecific antibodies: serenity before the storm
First of all, as far as the target is concerned, the bispecific antibody increases the selectivity of the target when the number of McAb targets is limited. For the dilemma that drug R&D enterprises generally face the shortage of targets, the combination of two targets can solve the problem to a certain extent, so that the pipeline of biopharmaceutical companies can be greatly expanded. Therefore, domestic pharmaceutical companies have been actively laying out this field.
Secondly, compared with McAbs, BsAb has comparable functions of target binding, inhibition, neutralization, carrying toxin, antibody-dependent cell cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). In addition, because it can be designed to combine “tumor target + immune cell target”, “tumor target + tumor target”, “immune target + immune target”, “inflammatory target + inflammatory target”, “vascular growth factor + tyrosine kinase receptor”, “virus target + virus target”, so it has the special function of connecting bridge between cells or proteins. More immune cells can be recruited to target tumor cells or multiple cross-linking inhibition on disease targets, so as to achieve a better therapeutic effect than monoclonal antibodies, or provide additional therapeutic options for diseases in which single target antibody drugs do not respond enough. It can achieve the therapeutic effect that can not be achieved by monoclonal antibody or monoclonal antibody combination therapy.
According to the relevant data, the anti-tumor effect of BsAb is 10-1000 times that of ordinary antibodies, and the lowest dosage can be reduced to 1/2000 of common antibodies. Because it is a promising field of cancer drug development, it also has a competitive advantage over general antibodies in efficacy and price.
In addition to tumor indications, scientists are also constantly exploring the therapeutic potential of bispecific antibodies in other diseases, such as HIV infection, viral and bacterial infection, osteoporosis, diabetes, autoimmune deficiency and so on.
As a cutting-edge technology, bispecific antibodies still have many challenges in the reasonable evaluation of animal models and preclinical research strategies. However, with the progress of many clinical studies, the problems such as the verification of different effect mechanisms of bispecific antibody projects have been gradually solved, and bispecific antibodies can be expected to provide new opportunities for the design and development of new drugs.
Reference
1. Li, Heliang, Phei Er Saw, and Erwei Song. “Challenges and strategies for next-generation bispecific antibody-based antitumor therapeutics.” Cellular & molecular immunology 17.5 (2020): 451-461.
