Blinatumomab is a CD19/CD3 bispecific antibody that is the first bispecific T cell engager (BiTE) and the first CD19-specific antibody approved by the U.S. Food and Drug Administration (FDA). As an immunotherapy drug, Blinatumomab selectively targets CD19 proteins that bind to the surface of hyperproliferative B-cell lymphoblasts, and specifically bind to CD3 proteins on the surface of T cells, thereby activating T cells to identify and kill hyperproliferating B cell lymphocytes. In the United States, each year approximately 6,000 people are diagnosed with extremely refractory acute lymphoblastic leukemia (ALL). Blinatumomab has passed the FDA’s rapid approval, which is a landmark event in the history of immunotherapy.
Development of BiTE technology
Initially, hybridization technology was proposed by Kohler and Milstein. In 1985, muromonab-CD3 (OKT3), the first monoclonal antibody (mAb) that specifically binds to human CD3, was developed and approved for the treatment of organ transplant rejection; In 1997, rituximab was approved as the first anti-tumor mAb to treat CD20(+) non-Hodgkin’s lymphoma (NHL). The success of these drugs has contributed to the development of antibody technology in the depths and various new technologies in an endless stream.
The concept of T cell-binding antibodies was first proposed by Staerz et al. One arm of a hybridoma antibody binds to CD3 and the other arm binds to an antigen target, thus forming a T cell-binding antibody. On one hand, a hybridoma antibody that binds to CD3 can activate T cells, and on the other hand, when the antibody binds to an antigen target, the activated T cells can destroy the antigen-carrying cells. This strategy has upgraded cancer immunotherapy to accurately track “enemies.”
In the 1980s and 1990s, a new bispecific antibody (bsAb) technique was proposed, and Peter Kufer et al. developed the incorporation of two or more single-chain variable fragments (scFv) in tandem with each other. Compared to common antibodies, the volume and molecular weight of a peptide chain are greatly reduced, called a bispecific T-cell engager, referred to as BiTE.
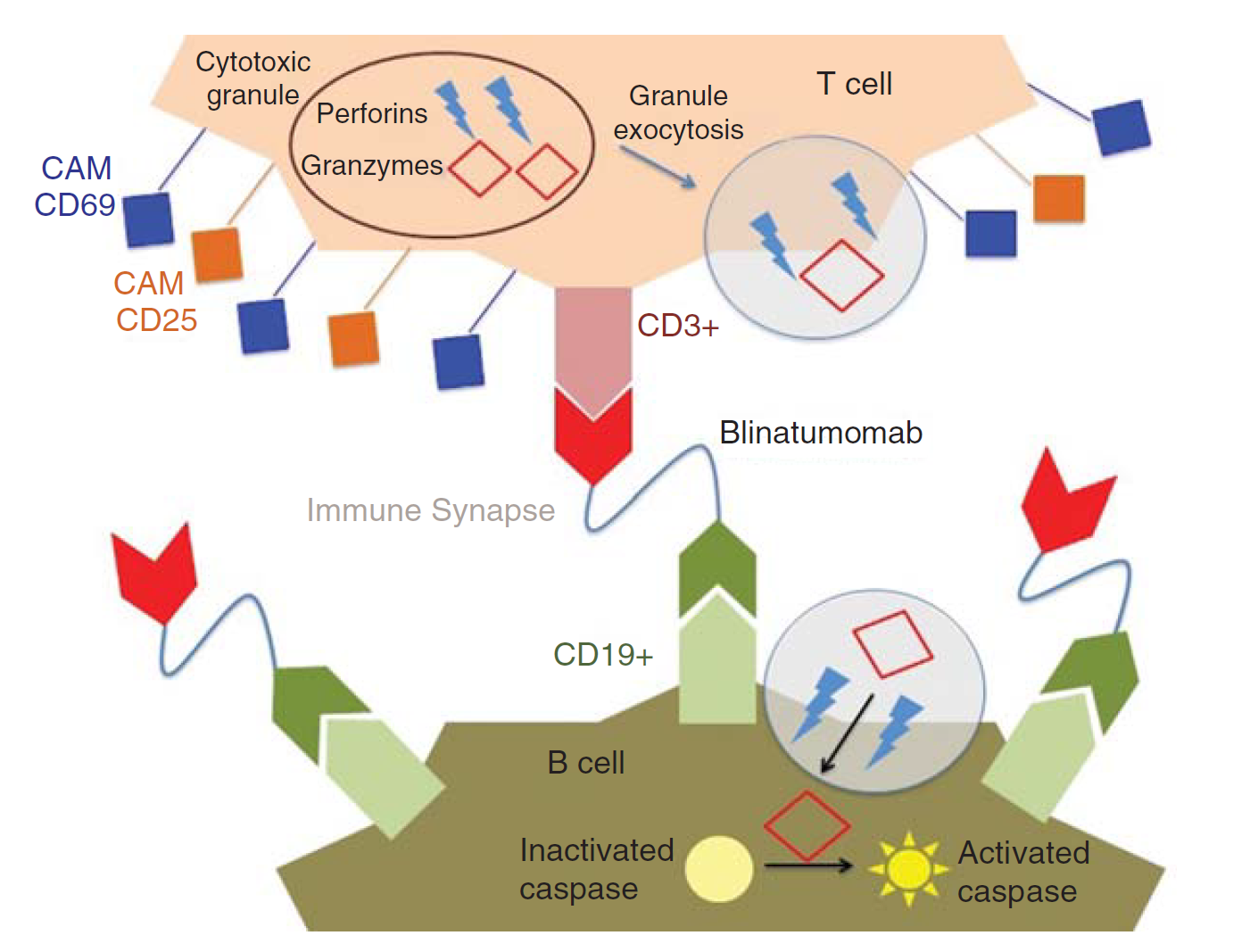
Fig.1 Mechanism of action of blinatumomab. (Rogala, 2015)
R&D of Blinatumomab
CD19 is a specific B cell plasma membrane protein involved in the regulation of the PI3/AKT pathway and plays a crucial role in the proliferation and differentiation of B cells. CD19 is rarely expressed in plasma cells, but is continuously expressed during the proliferation of B cells, which explains that CD19 is selected as a target for antibody targeting B cells.
In 1998, Micromet developed three bispecific antibodies, namely MT103, blinatumomab (INN) and AMG 103. Blinatumomab is an aglycosylated murine antibody containing two tandem scFvs. The linker of these two scFv fragments was derived from murine anti-human CD16 mAb HD37 and CD3 mAb OKT3. The anti-CD19 and anti-CD3 scFvs were linked in the variable region with Gly4Ser, respectively, and the two scFvs were ligated in series with Gly4Ser. In a single BiTE, the specific order of the variable heavy (VH) and variable light (VL) is critical for the activity of blinatumomab, and of the multiple VH/VL combinations, only one becomes a drug.
Conventional mAbs do not bind to T cells because T cells do not express FC-gamma receptors such as CD16, CD32 or CD64. Only specific antibodies that bind to T cell surface antigens can activate T cells, such as anti-CD3, CD28 and CD137, which continuously activate T cells, probably because Fc-γ receptor-positive immune cells present antibodies to T cells by a multivalent method. Due to the lack of an Fc-gamma domain, blinatumomab only has one arm binds to CD3 and the other arm binds to CD19.
Clinical trial evaluation of Blinatumomab
A multicenter Phase I clinical trial investigated the tolerability and safety of Blinatumomab in patients with NHL. The trial showed that after administration, patients released cytokines and activated T cells, and reduced the persistence of B cells. In 2012, a multi-center phase II clinical trial was performed to demonstrate the therapeutic effect of Blinatumomab on refractory B-cell ALL, further evaluating the safety and efficacy of the drug, and determining the PK value of the drug. It showed that the patient’s hematologic recurrence-free survival rate was 61% after drug treatment, and in the hematopoietic stem cell transplantation subgroup the hematologic recurrence-free survival rate was 65%. This drug provides long-term complete remission in patients with persistent or refractory B-cell ALL. Phase III clinical trials have shown that the drug is superior to standard therapy in the treatment of R/R ALL.
After Phase III clinical trials, the U.S. Food and Drug Administration (FDA) approved the marketing of blinatumomab (trade name Blincyto®) in December 2014 for the treatment of relapsed or refractory Philadelphia chromosome-negative ALL, and inert B cell lymphoma. In addition, the FDA has also granted its breakthrough drug and priority review certification and orphan drug qualification. In 2015, the European Union (EU) also approved the listing of the drug.
Reference
1. Rogala, Britny, et al. “Blinatumomab: enlisting serial killer T-cells in the war against hematologic malignancies.” Expert opinion on biological therapy 15.6 (2015): 895-908.
