TG Therapeutics is a biopharmaceutical company dedicated to developing innovative treatments for patients with B-cell-mediated diseases. Recently, the company announced the launch of TG-1801 ‘s first human Phase I study, a new, pioneering, anti-CD47/CD19 bispecific antibody (bsAb) developed for the treatment of recurrent or refractory B-cell lymphoma.
TG-1801 is the first treatment that targets both CD19 and CD47. CD19 is a B cell-specific marker widely expressed in B cell malignant tumors, while CD47 is expressed in both healthy and tumor cells, transmitting the “don’t eat me” signal. In order to escape macrophage-mediated phagocytosis, CD47 is widely expressed in normal cells, including red blood cells and platelets. Co-targeting CD47 and CD19,TG-1801 has the potential to overcome the limitations of existing CD47 targeting therapy and avoid the side effects of indiscriminate blocking of CD47 on healthy cells.
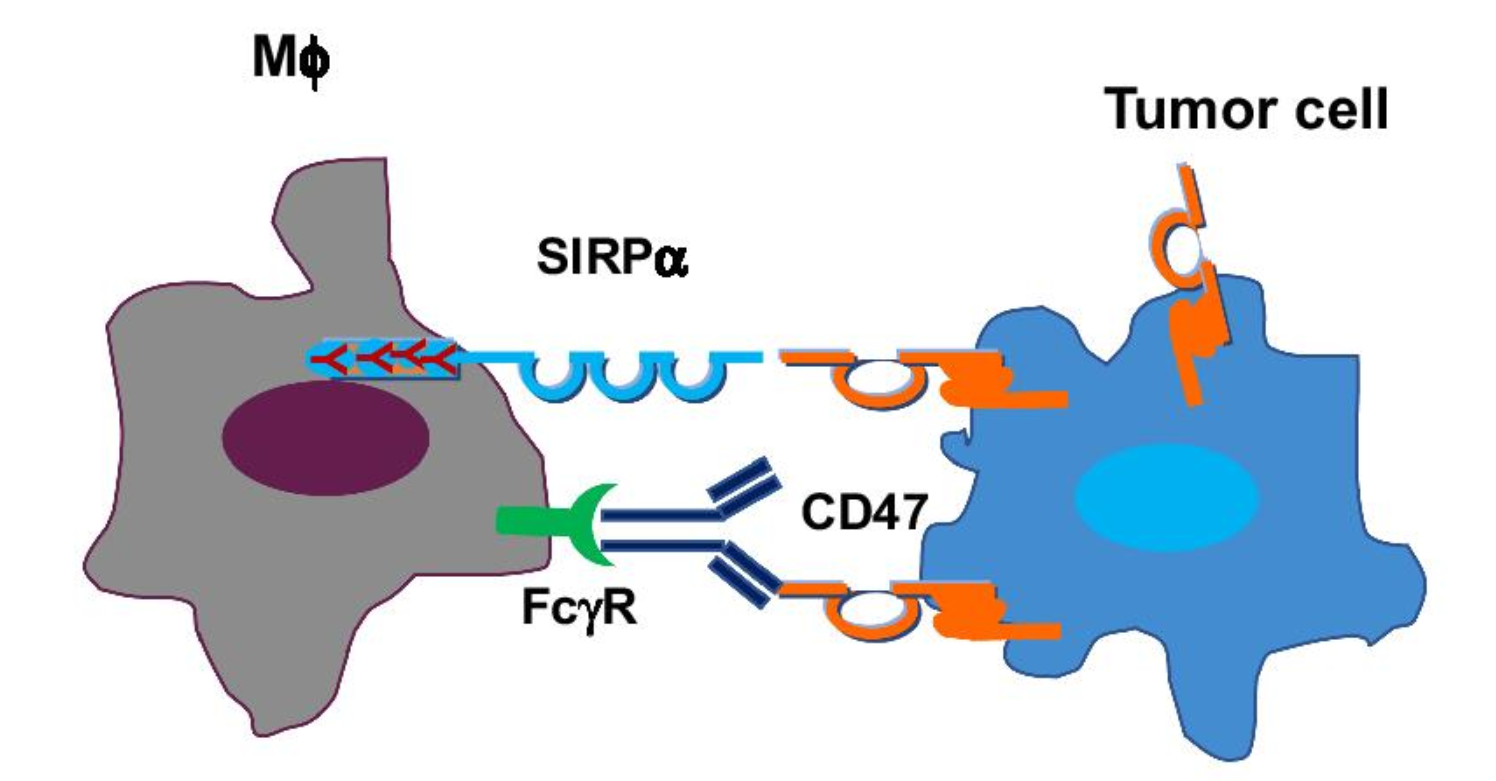
Fig. 1 “Don’t eat me” signal versus “eat me” signal. (Jiang, 2021)
The open-label, multicenter Phase I study was designed to assess the safety, pharmacokinetics, efficacy, and recommended II dose of TG-1801. The main purpose of the study was to determine the maximum tolerable dose of (MTD), and to describe the safety characteristics of TG-1801, with secondary endpoints including pharmacokinetic characteristics and preliminary anticancer activity.
Michael S.Weiss, Executive Chairman and Chief Executive Officer of TG, said, “We are very pleased to announce the launch of the human clinical development of TG-1801, a proprietary, first anti-CD47/CD19 bispecific antibody we obtained from Novimmune last year. CD47 targeted therapy has produced promising early clinical results, and we look forward to exploring the potential of this dual-target immunotherapy, with the long-term goal of combining TG-1801 with our other internal immunotherapies and targeted drugs. To create new treatment options for patients with B-cell carcinoma. “
At present, TG has two therapies into III clinical development: (1) ublituximab, a new type of glycosylated anti-CD20 monoclonal antibody, Targeting specific epitopes of CD20 antigen on mature B lymphocytes; (2) umbralisib, an oral, daily, new generation of PI3K δ inhibitors, can uniquely inhibit CK1- ε, which may enable it to overcome some tolerance problems associated with the first generation of PI3K δ inhibitors.
The combination of ublituximab, umbralisib and U2 is in the clinical stage of III in the treatment of hematological malignant tumors, in addition, the treatment of multiple sclerosis with ublituximab has also been in the clinical stage of III. Just recently, the company pushed its anti-PD-1 monoclonal antibody TG-1501, a covalently bound BTK inhibitor TG-1701, into Phase I clinical practice.
In January, the FDA in the United States granted umbralisib a breakthrough drug qualification (BTD) for adult patients with marginal zone lymphoma (MZL) who had previously received at least one anti-CD20 regimen.
Reference
1. Jiang, Zhongxing, et al. “Targeting CD47 for cancer immunotherapy.” Journal of Hematology & Oncology 14.1 (2021): 1-18.
