Using human tissue from a phase 1 clinical trial, researchers have demonstrated that treatment with GB1275, an oral small-molecule CD11b agonist, can activate the STING and STAT1 signaling pathways in human tumor-associated macrophages (TAMs). Furthermore, the combination of GB1275 with chemotherapy or radiation therapy can amplify the STING/IFN signaling in pancreatic cancer.
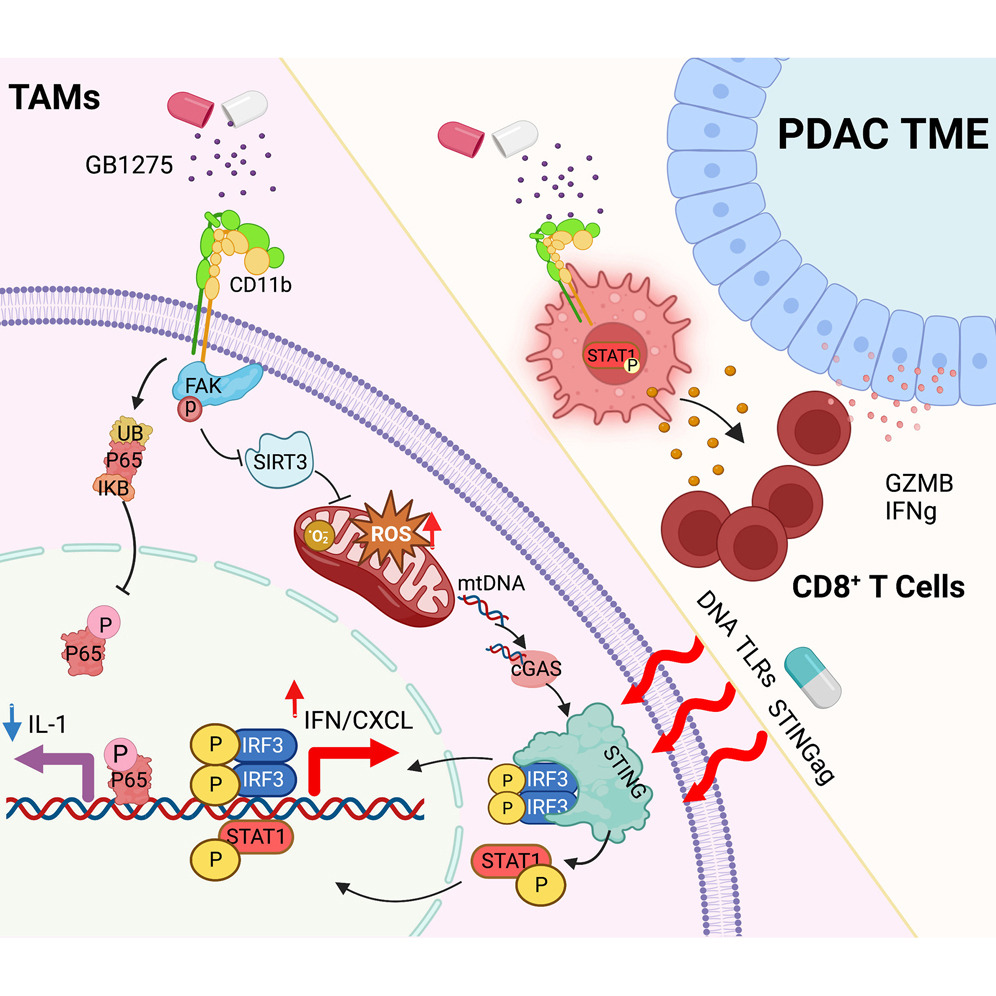
Fig. 1 Context-dependent activation of STING-interferon signaling by CD11b agonists enhances anti-tumor immunity (Liu, 2023)
Pancreatic cancer, specifically pancreatic ductal adenocarcinoma (PDAC), is a highly malignant and challenging digestive tract malignancy, accounting for over 95% of all pancreatic cancers.
In recent years, the incidence and mortality rates of pancreatic cancer have been increasing. Early diagnosis of pancreatic cancer is difficult, and it is often detected at an advanced stage when the cancer cells have already spread. Although some chemotherapy drugs are effective, they often lead to drug resistance, and immune-based therapies have limited efficacy. The 5-year survival rate for pancreatic cancer is less than 7%, making it one of the deadliest malignancies, often referred to as the “king of cancers.”
The presence of tumor-associated macrophages (TAMs) is associated with poor clinical outcomes in many types of cancer. However, TAMs exhibit significant plasticity and can play both pro-tumor and anti-tumor roles during treatment. In certain solid tumors, such as pancreatic ductal adenocarcinoma (PDAC), the dense stromal fibrosis can push TAMs towards an immunosuppressive and damage repair phenotype. Reprogramming TAMs into an anti-tumor phenotype is an appealing therapeutic strategy for such cancers.
On May 25, 2023, researchers from Washington University in St. Louis published a study titled “Context-dependent activation of STING-interferon signaling by CD11b agonists enhances anti-tumor immunity” in the journal Cancer Cell.
The study revealed the mechanism of CD11b agonist treatment for pancreatic cancer. CD11b agonists activate the STING-interferon signaling pathway, enhancing anti-tumor immunity. When combined with chemotherapy or radiation therapy, they generate a stronger anti-tumor immune response.
Anti-cancer macrophages are characterized by high expression of tumor necrosis factor-alpha (TNF-α), IL-12a, inducible nitric oxide synthase, or MHC molecules, as well as chemokines, like CXCL9 and CXCL10. On the other hand, protumoral macrophages exhibit high expression of IL-10, IL-1 decoy receptor, IL-1Rα, arginase-1, as well as CD163, CD204, or CD206.
In human pancreatic ductal adenocarcinoma (PDAC), tumor-associated macrophages (TAMs) are abundant in the tumor tissues of many patients. In preclinical animal models, slowing down PDAC progression and improving its response to various drugs has been achieved by reducing TAMs through the blockade of key chemokine receptors (such as CCR2 or CXCR1/2) or inhibition of the colony-stimulating factor 1 receptor (CSF1R). However, these approaches have not shown significant clinical efficacy in solid tumors so far.
This lack of efficacy in humans may be due to significant compensation by non-targeted subpopulations of myeloid cells, which ultimately limits their therapeutic effectiveness. Therefore, researchers have shifted their focus to reprogramming TAMs to support anti-tumor immune therapies.
Previous studies have identified CD11b as a novel target for tumor immunotherapy. In mouse models of PDAC and other cancers, small-molecule CD11b agonists have shown moderate reduction in the number of tumor-infiltrating immunosuppressive bone cells (such as TAMs, monocytes, and granulocytes), leading to increased T cell-mediated tumor control. These experimental findings have prompted CD11b agonists into early clinical trials for solid tumors using CD11b agonists.
In this latest study, the research team investigated the cellular and molecular mechanisms underlying the induction of anti-tumor immunity by CD11b agonists and identified potential directions for combination therapy.
The study revealed that CD11b agonists alter the phenotype of TAMs by simultaneously inhibiting NF-κB signaling and activating interferon gene expression. Inhibition of NF-κB signaling involves the degradation of the p65 protein, while CD11b agonist-induced effects on mitochondrial dysfunction through FAK-mediated pathways induce STING/STAT1 pathway-mediated interferon gene expression. The magnitude of this effect depends on the tumor microenvironment and is amplified by cytotoxic therapy.
Using human tissue samples from a phase 1 clinical trial, the research team demonstrated that treatment with GB1275 (an oral small-molecule CD11b agonist) can activate the STING and STAT1 signaling pathways in human TAMs. Moreover, combining GB1275 with chemotherapy or radiation therapy can enhance the STING/IFN signaling, leading to a more potent immune therapeutic effect.
These findings provide insight into the potential mechanisms of CD11b agonists in the treatment of pancreatic cancer and identify the patient population that is more likely to benefit from this therapy.
Reference
1. Liu, Xiuting, et al. “Context-dependent activation of STING-interferon signaling by CD11b agonists enhances anti-tumor immunity.” Cancer Cell 41.6 (2023): 1073-1090.
