In a recent study, researchers from Stanford University in the United States collected data from previous studies on CAR-T cell therapy to investigate the reactivation of human herpesvirus 6 (HHV-6) in patients undergoing chimeric antigen receptor (CAR) T cell therapy. The study aimed to describe the characteristics of HHV-6 reactivation, especially in the context of CAR-T cell therapy for B-cell lymphoma or leukemia. The researchers found that CAR-T cell therapy could potentially lead to the reactivation of HHV-6 in patients. The relevant research findings were published in the November 16, 2023, issue of the Nature journal under the title “Latent human herpesvirus 6 is reactivated in CAR T cells.”
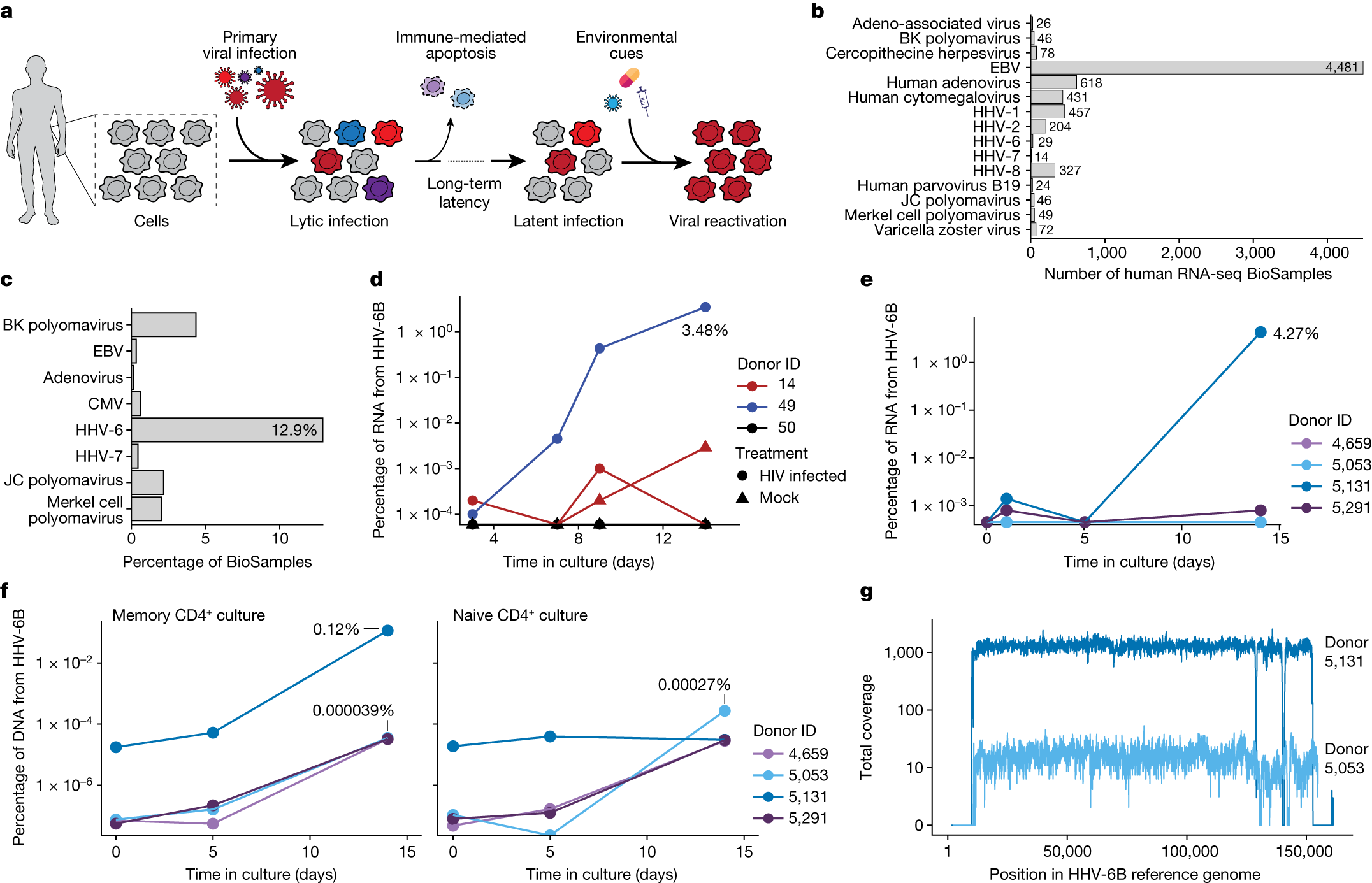
The authors reanalyzed the single-cell RNA sequencing (scRNA-seq) datasets of three groups of patients treated with autologous CAR-T cell products. While HHV-6 virus transcripts were not detected in CAR-T cell products before infusion, a significant increase in HHV-6+ cells was observed in post-infusion samples.
The authors found that 28 CAR-T cells in post-infusion samples expressed HHV-6B transcripts, with 13 CAR-T cells resembling HHV-6 “super-expressor” cells, a rarity in research-grade allogeneic CAR-T cells. Detailed temporal analysis of two patients with HHV-6 super-expression showed the appearance of HHV-6+ cells one week after treatment, aligning with clinical symptoms of immune effector-related neurotoxicity syndrome. Symptoms such as confusion and decline in neurocognitive abilities matched the presence of HHV-6 virus in the blood, though causation was not established for all patients.
These findings suggest a connection between T cell activation, proliferation, and culture duration and the reactivation of HHV-6, emphasizing the need for further consideration in the development and monitoring of CAR-T cell therapy.
The new study also identified HHV-6B reactivation events in standard CD4+ T cell cultures, confirming a potential association between cell therapy products and lytic HHV-6 infection, a correlation previously reported in clinical trials of CAR-T cell therapy.
HHV-6B infection occurs in nearly 70% of individuals before the age of three, leading to a common childhood illness—subacute erythematous rash or infantile roseola. By adulthood, approximately 95% of people have been infected with this virus. During the primary infection period, the virus is present in various cell and tissue types, and viral DNA continues to survive in peripheral blood mononuclear cells after infection.
The authors emphasize the role of comprehensive genomic analysis in identifying cell therapy products as potential sources of virus infection. The correlation between HHV-6 reactivation and CAR-T cell therapy suggests the possibility of other latent viruses reactivating in various cell therapies, prompting the need for further research.
Reference
1. Lareau, Caleb A., et al. “Latent human herpesvirus 6 is reactivated in CAR T cells.” Nature 623.7987 (2023): 608-615.