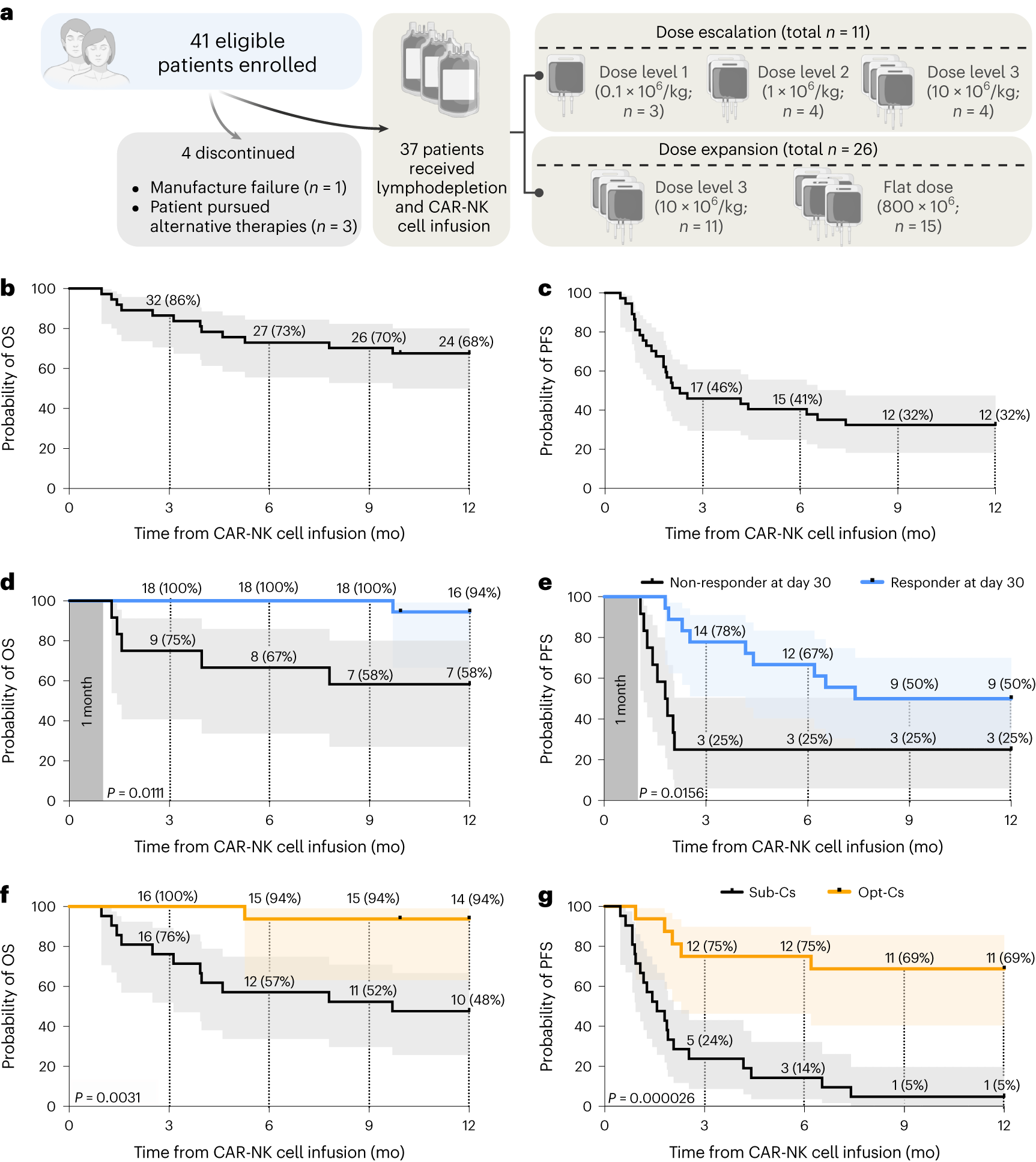
In a recent study, researchers from the University of Texas MD Anderson Cancer Center in the United States reported favorable results from a Phase I/II clinical trial involving 37 patients with relapsed or refractory B cell malignancies. The patients received targeted CD19-specific cord blood-derived chimeric antigen receptor (CAR) natural killer (NK) cell therapy. The results showed an overall response rate of 48.6% after 100 days of treatment, with one-year progression-free survival and overall survival rates of 32% and 68%, respectively. The clinical trial, emphasizing excellent safety with no severe cytokine release syndrome, neurotoxicity, or graft-versus-host disease, was published online in the Nature Medicine journal on January 18, 2024, titled “Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19+ B cell tumors: a phase 1/2 trial.”
Another crucial finding from the clinical trial was the significance of donor selection criteria for allogeneic cord blood-derived CAR-NK cell manufacturing. Cord blood units frozen within 24 hours of collection and units with low nucleated red cell content were associated with significantly better outcomes. CAR-NK cells derived from these units demonstrated a one-year progression-free survival rate of 69% and an overall survival rate of 94%. In contrast, CAR-NK cells produced from units with higher nucleated red cell content or longer collection-to-freezing times had a one-year progression-free survival rate of 5% and an overall survival rate of 48%.
Dr. Katy Rezvani, the corresponding author and professor of Stem Cell Transplantation and Cellular Therapy at the University of Texas MD Anderson Cancer Center, stated, “The responses observed in these patients are very encouraging as we continue to evaluate the long-term efficacy of CAR-NK cell therapy for these malignancies. To achieve successful allogeneic cell therapy, we must also identify characteristics of the optimal cord blood donor for CAR-NK cell manufacturing. We were able to identify two key factors associated with cord blood units most likely to yield a positive clinical response and uncover the biological mechanisms behind this phenomenon.”
The study also highlighted encouraging response rates for different types of B cell malignancies. Patients with low-grade non-Hodgkin lymphoma achieved a 100% overall response rate 30 days post-treatment, while those with untreated chronic lymphocytic leukemia had a 67% overall response rate, and diffuse large B-cell lymphoma (DLBCL) patients had a 41% overall response rate.
The researchers observed durable responses to CAR-NK cell therapy. One year after treatment, 83% of low-grade non-Hodgkin lymphoma patients, 50% of chronic lymphocytic leukemia patients, and 29% of DLBCL patients achieved a complete response. Patients who responded 30 days after treatment had significantly increased chances of progression-free survival one year later.
Rezvani commented, “Our research underscores the importance of identifying predictive indicators of response after donor-specific allogeneic cell therapy, particularly as one donor may be used to treat hundreds of patients. CAR-NK cells have the potential for advanced manufacturing and storage for immediate use, increasing patients’ chances to access these cell therapies, reducing treatment time, and lowering treatment costs.”
The selection criteria identified in this study are being applied in clinical trials at the University of Texas MD Anderson Cancer Center, where genetically modified cord blood NK cells are used to target other antigens and malignancies, including solid tumors.
Reference
1. Marin, David, et al. “Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19+ B cell tumors: a phase 1/2 trial.” Nature Medicine (2024): 1-13.