T-cell receptor-engineered T cells (TCR-T) represent a form of cellular immunotherapy and a subtype of adoptive cell transfer (ACT). It involves the identification and selection of TCR sequences capable of specifically binding target antigens, genetically engineering T cells from the patient’s peripheral blood to express these selected TCRs, and then reinfusing these modified T cells back into the patient. This enables the T cells to selectively recognize and eliminate tumor cells expressing the target antigens, thereby achieving therapeutic goals in cancer treatment.
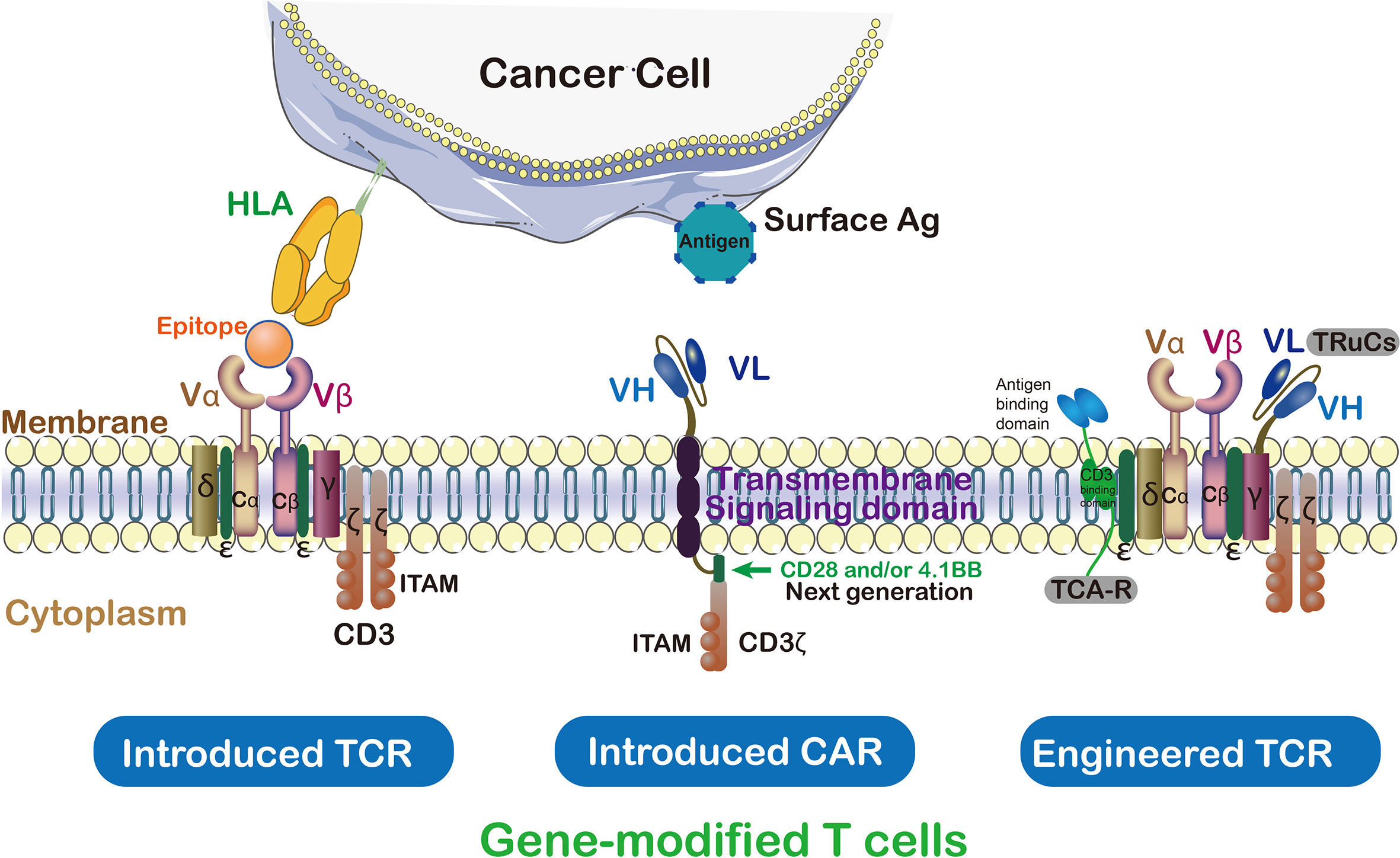
Comparison between TCR-T and CAR-T
Some TCR-T products have been utilized domestically and internationally for treating patients with refractory/recurrent melanoma, synovial sarcoma, multiple myeloma, lung cancer, among others, showing promising safety profiles and efficacy in certain clinical trial settings. Compared to CAR-T therapy, TCR-T demonstrates superior penetration into solid tumors, enhanced specificity towards tumor cells, and a lower risk of immune rejection and antibody generation.
The primary distinction between CAR-T and TCR-T lies in how they recognize tumor cells. CAR-T involves the genetic engineering of T cells to express chimeric antigen receptors (CARs), allowing them to recognize non-MHC (major histocompatibility complex) restricted antigens on the surface of tumor cells. Conversely, TCR-T modifies the natural T cell receptors on T cells to recognize MHC-restricted antigens on the surface of tumor cells.
A key advantage of TCR-T therapy lies in its ability to target MHC-restricted antigens on tumor cells, which are often highly expressed on the surface of tumor cells, enabling TCR-T cells to more accurately identify and attack tumor cells. Additionally, TCR-T therapy exhibits lower immunogenicity since it utilizes naturally occurring T cell receptors rather than artificially synthesized CARs, thus less likely to elicit immune responses.
TCR-T Technologies
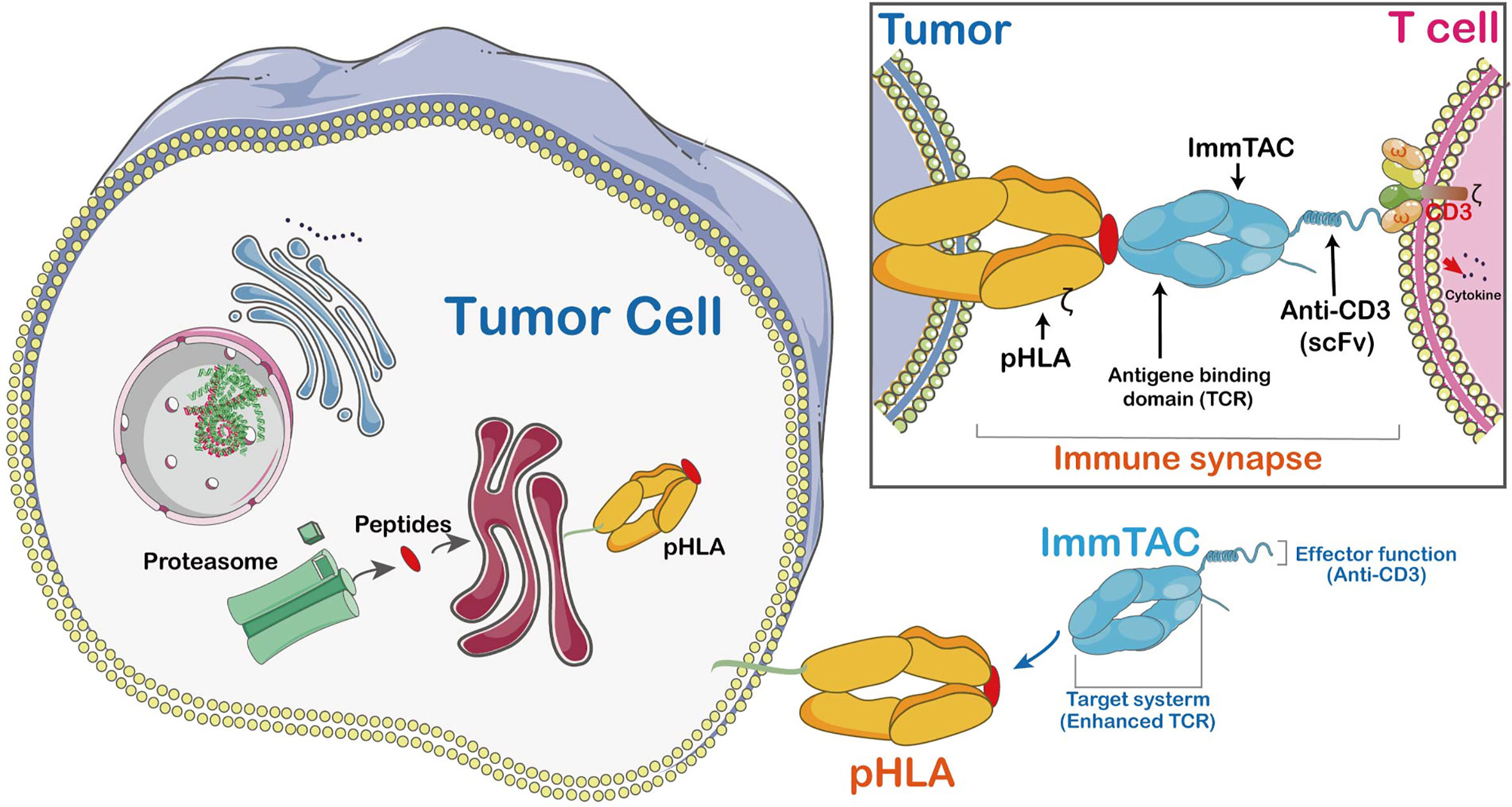
In recent years, numerous innovations have emerged in T-cell receptor (TCR) engineering technologies aimed at improving T cell recognition of specific tumor antigens and treatment efficacy. For instance: Immune mobilizing monoclonal TCRs Against Cancer (ImmTAC) are fusion proteins combining engineered TCRs with single-chain antibody fragments to enhance T cell persistence and anti-tumor activity. Unlike antibodies targeting surface or secreted proteins alone, ImmTACs’ TCRs can recognize intracellular target peptides presented by HLA.
T-cell Receptor Fusion Constructs (TRuCs) fuse antibody-based binding domains with TCR subunits, presenting novel target specificity and HLA-independent capabilities, thereby precisely identifying tumor surface antigens. TRuCs integrate into T cell surface TCR complexes, retaining their activation and effector functions, with superior anti-tumor effects compared to second-generation CAR-T cells. Additionally, TRuCs predominantly utilize TCR signaling while CARs employ limited CD3z signaling.
T cell Antigen Couplers (TACs) constitute an MHC-independent platform enhancing anti-tumor responses and reducing toxicity by combining CD3 domain with TCR. TAC activity relies on the selection of CD3 binding domains, where subtle differences may lead to significant functional outcomes. Compared to second-generation CARs, TAC-engineered T cells demonstrate advantages in solid tumor infiltration, reduced expansion of healthy tissue-resident T cells, and off-tumor toxicity reduction.
Advancements in TCR-T Research
Reducing TCR mispairing to enhance expression and functionality
TCR-T therapy relies on introducing TCR genes with tumor reactivity into T cells via mRNA or viral transduction to redirect T cells specifically towards tumor cells. However, the presence of endogenous TCR genes may lead to mispairing, posing safety risks such as graft-versus-host disease (GvHD). Several strategies have been explored to reduce mispairing, including introducing inter-chain disulfide bonds, substituting human TCR constant domains with mouse sequences, manipulating TCR constant domains, generating single-chain TCRs (scTCRs), and genetically editing out endogenous TCRs. Furthermore, gene editing technologies like CRISPR/Cas9 hold potential in TCR-T therapy but need to address challenges such as off-target toxicity.
- Enhancing T cell persistence
T cell persistence is crucial for immune surveillance, yet tumor-specific T cells infused into non-responding patients often lack persistence. To enhance T cell persistence, researchers are actively exploring combinations of various cytokines, some of which have entered clinical trial stages. Additionally, preconditioning regimens such as lymphodepletion can conserve limited cytokines, eliminate immunosuppressive cells like Tregs and MDSCs, and enhance T cell engraftment and expansion. Moreover, selecting less differentiated T cell subsets like Tscm and Tcm cells, modified with CARs to produce antigen-specific T cells, can also enhance persistence and functionality, mediating strong and enduring anti-tumor responses.
- Optimizing T cell migration
Effective migration and infiltration of tumor-specific T cells into solid tumors rely on the interaction between tumor-secreted chemokines and chemokine receptor expression on T cells. Designing T cells to express specific chemokine receptors is crucial for enhancing this process’s efficiency. By expressing receptors like CCR2, CXCR2, CCR4, and CXCR4 on Tc cells, significant improvement in T cell homing and localization to tumors can be achieved, thereby enhancing their anti-tumor activity. These strategies demonstrate immense potential and clinical prospects in the field of tumor immunotherapy. Additionally, combining with radiotherapy and chemotherapy can further promote T cell homing and infiltration. While approved cancer chemokine immunotherapy strategies are yet to be established, multiple studies show promising clinical potential.
- Overcoming the tumor immune microenvironment
Tumor cells reside in a complex microenvironment consisting of various immunosuppressive cells, factors, and stroma, collectively supporting tumor growth, migration, metastasis, and evasion from immune system attacks. To improve this immunosuppressive environment, various strategies have been explored. Direct use of immune checkpoint inhibitors such as anti-PD-1, anti-PD-L1, and anti-CTLA-4 antibodies can enhance immune responses, although challenges of resistance and toxicity remain.
To overcome these challenges, researchers utilize chimeric switch receptors (CSRs) to convert immune inhibitory signals within T cells into stimulatory signals, such as PD-1:CD28 combinations, enhancing T cell anti-tumor effects. Meanwhile, blockade of immune inhibitory factors such as TGF-β also enhances immunotherapy efficacy. These strategies demonstrate the potential to convert immune suppression into stimulatory signals, providing new directions for tumor immunotherapy.
- Developing new targets
The application of TCR-T cell therapy in cancer is limited by effective and safe antigen targets. Currently, tumor-associated antigens are predominantly used, but they may lead to autoimmune toxicity. Truly tumor-specific antigens and novel antigens present safer alternatives. Personalization and heterogeneity of novel antigens pose challenges in clinical development. With advancements in sequencing technologies, personalized TCR-T immunotherapy targeting novel antigens may become mainstream in the future. “Hot” tumors with higher TIL content are sensitive to checkpoint blockade therapy, and combining therapies can enhance the efficacy of TCR-T cell therapy. Newly identified tumor-associated antigens also offer potential targets for future TCR-T therapy.
Creative Biolabs masters the most advanced CAR/TCR technology. With state-of-art TCR development platforms and advanced technologies, Creative Biolabs is capable of offering a broad range of TCR-T therapy development services, including TCR engineered T cell biomarker identification and selection, design, construction, and analysis.
Reference
1. Zhao, Qijie, et al. “Engineered TCR-T cell immunotherapy in anticancer precision medicine: pros and cons.” Frontiers in immunology 12 (2021): 658753.