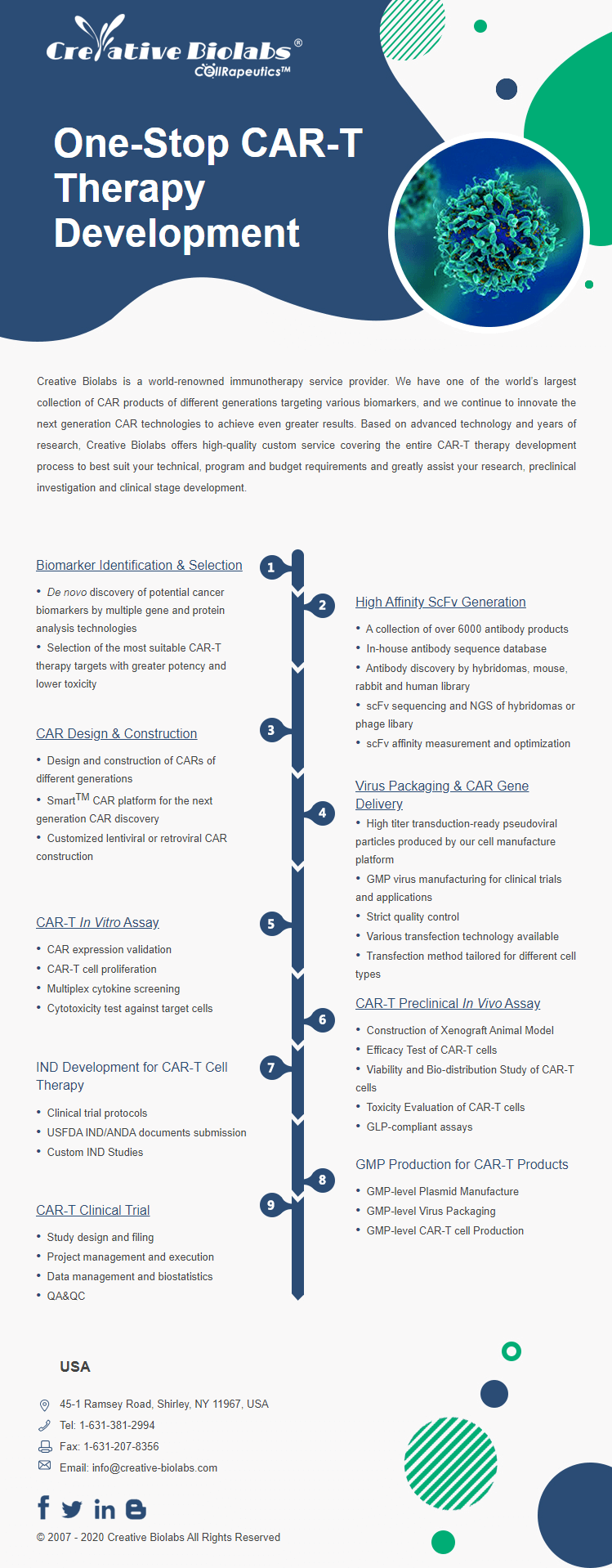
One-Stop CAR-T Therapy Development Solutions
Your End-to-End Partner in CAR-T Therapy Development. Streamline your pipeline, de-risk your program, and expedite your path to IND.
Streamlining CAR-T Development from Discovery to Delivery, Together.
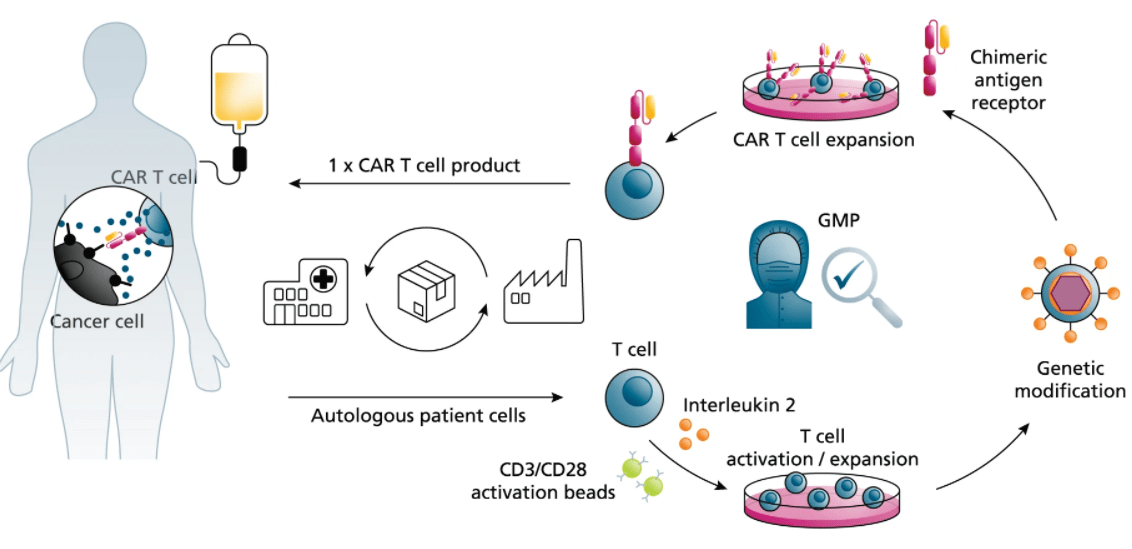
The development of Chimeric Antigen Receptor (CAR) T-cell therapy is a complex, multi-stage process demanding deep scientific expertise, specialized technologies, and rigorous quality control. Navigating this journey from target discovery to a clinical candidate involves significant time, resources, and risk.
As a leading Contract Research Organization (CRO), we provide a seamless, integrated suite of services designed to support and accelerate every phase of your CAR-T program. Our "One-Stop Solution" eliminates the need for multiple vendors, ensuring consistency, efficiency, and clear communication. Partner with us to transform your innovative concepts into life-saving therapies.
Our Full-Spectrum CAR-T Service Portfolio
Modular and fully integrated solutions for your program: choose individual services or partner with us for a complete, end-to-end development plan.
CAR-T Assay & Development Hub
From comprehensive assays to specialized production, explore our end-to-end service capabilities.

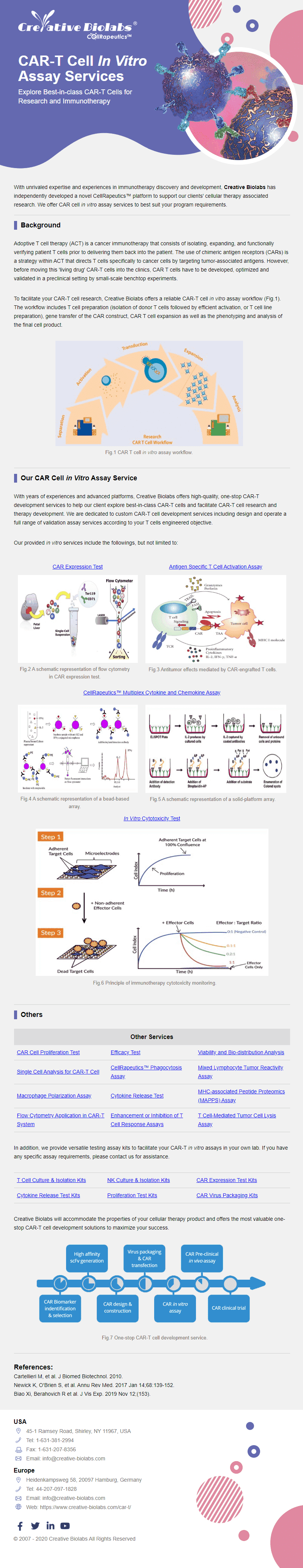
CAR Cell In Vitro Assay
- ✓CAR Expression Test
- ✓Cytokine Release Test
- ✓Cytotoxicity Test
- ✓Proliferation Test
- ✓T Cell-mediated Tumor Cell Lysis Assay
- ✓Single Cell Analysis
- ✓Multiplex Cytokine & Chemokine Assay
- ✓Mixed Lymphocyte Tumor Reactivity Assay
- ✓MAPPS Assay
- ✓TCR Assay
- ✓Flow Cytometry Application
- ✓T Cell Response Detection
- ✓Phagocytosis Assay
- ✓Antigen Specific T Cell Activation
- ✓CAR-T/NK & TCR-T Cell Avidity Analysis
- ✓Macrophage Polarization Assay
- ✓CAR-T In Vitro Efficacy Assays
- ✓Anti-CAR Cell Exhaustion Engineering
Core Development & Production
Next-Generation Platforms
Specialized Applications
Advanced Engineering & Discovery

CAR Cell In Vitro Assay
- ✓CAR Expression Test
- ✓Cytokine Release Test
- ✓Cytotoxicity Test
- ✓Proliferation Test
- ✓T Cell-mediated Tumor Cell Lysis Assay
- ✓Single Cell Analysis
- ✓Multiplex Cytokine & Chemokine Assay
- ✓Mixed Lymphocyte Tumor Reactivity Assay
- ✓MAPPS Assay
- ✓TCR Assay
- ✓Flow Cytometry Application
- ✓T Cell Response Detection
- ✓Phagocytosis Assay
- ✓Antigen Specific T Cell Activation
- ✓CAR-T/NK & TCR-T Cell Avidity Analysis
- ✓Macrophage Polarization Assay
- ✓CAR-T In Vitro Efficacy Assays
- ✓Anti-CAR Cell Exhaustion Engineering
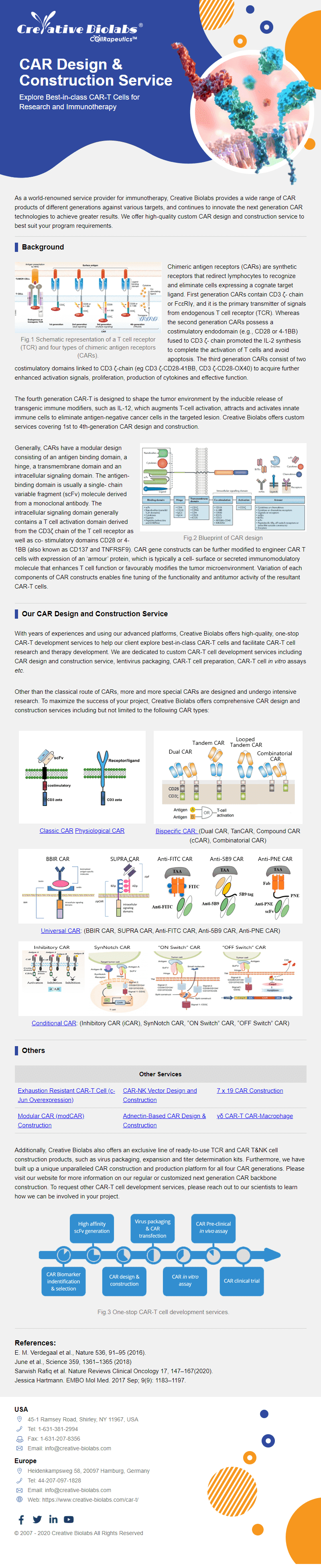
Platforms for Next-Generation CAR-T Engineering
Precision engineering for the next wave of CAR-T, providing you with advanced tools for superior therapeutic design and control.
Smart™ CAR Construction
Going beyond classical CAR-T generations, the Smart™ CAR platform offers next-generation designs like Dual CARs, TriCARs, and Logic-gated CARs. This allows for targeting multiple antigens and introducing novel regulatory mechanisms to create more specific, personalized therapies that overcome tumor evasion and off-target toxicities.
Learn more
mRNA-Based CAR Cell Platform
This platform uses transient mRNA expression for a safer, better-controlled cell therapy. By avoiding viral vectors, it minimizes risks like insertional mutagenesis. The temporary nature of mRNA expression means adverse effects are not long-lasting, making it ideal for patients needing short-term, reversible immune modulation without permanent genomic changes.
Learn more
CRISPR-edited CAR Cell Technology
CRISPR offers efficient, precise T-cell editing to improve therapeutic efficacy. It enables the creation of allogenic "universal" CAR-T cells by knocking out endogenous genes, increasing accessibility. It can also ablate inhibitory receptors to enhance persistence and integrate CAR cassettes at specific sites for controlled expression, enabling highly personalized therapies.
Learn moreCase Study: CAR-T In Vitro Assay
A comprehensive analysis showcasing the manufacturing, characterization, and cytotoxic efficacy of our anti-CD19 CAR-T cells.
CAR-T Production
Activated T cells were transduced with the lentivirus carrying the anti-CD19 CAR construct... The percentage of CAR-positive cells after enrichment and expansion (day 7-10) was determined using flow cytometry (27.63% for the first batch; 61.57% for the second batch). Analysis of CAR-T showed 30-40 fold expansion.
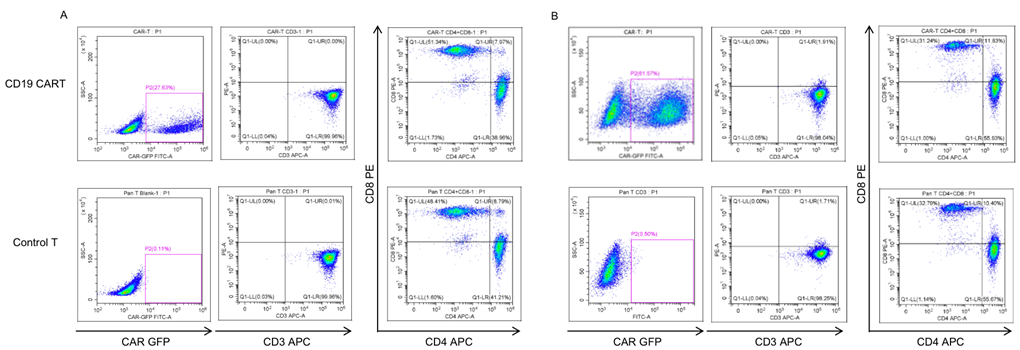
Fig. 2 Characterization of first batch primary CAR-T (A) and second batch primary CAR-T (B).
CAR-T Cell In Vitro Characterization
The cell viability was analyzed for CAR-T cells after thawing. The average cell recovery after thawing was 70%. 2-3 days after thawing, the CAR-T cells were characterized using FACS. Cryopreservation did not affect the frequency of CAR+ T cells, CD3+ T cells or the CD4+/CD8+ subpopulation composition.
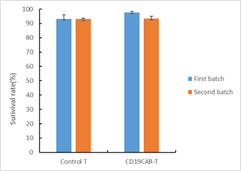
Fig. 3 Cell viability analysis of frozen CAR-T cells after thawing.
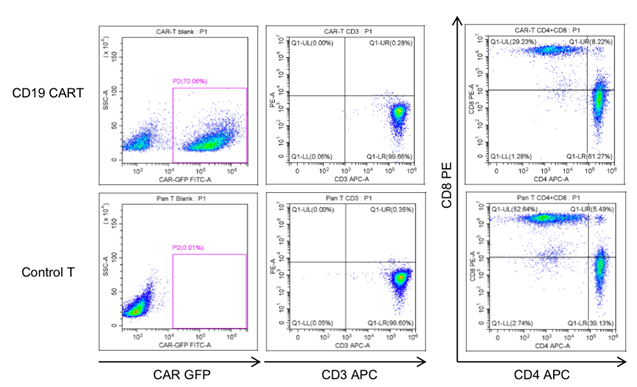
Fig. 4 Characterization of frozen CAR-T cells after thawing.
CAR-T In Vitro Cytotoxicity Test
The killing of CD19+ CAR-T cells against K562-CD19+ target cells were determined by using FACS. The target cells were co-cultured with CD19 CAR-T cells and control T cells at effector to target (E:T) ratio (2:1).
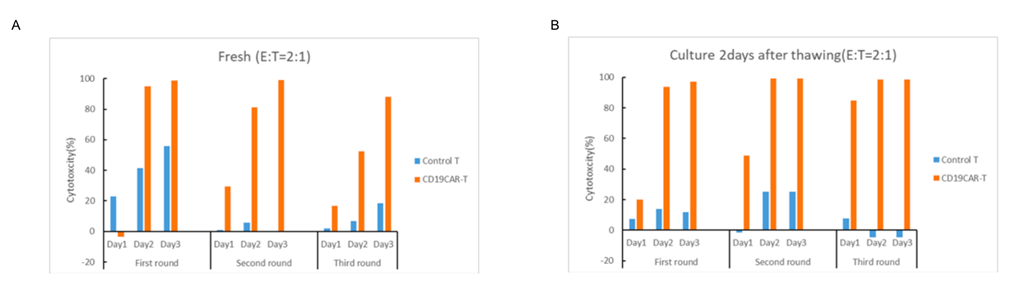
Fig. 5 In vitro cytotoxicity assay of fresh CAR-T and frozen CAR-T cells after thawing.
The CAR-T Development Toolkit
A complete toolkit containing high-quality reagents, advanced CAR vectors, and custom-designed products to support and streamline your research workflow.
Our Product Portfolio
View All Product Portfolios
Custom CAR Products
We have developed a proprietary online system for customizing CAR and CAR cell products, which offers full options to meet all unique needs. The customization process can be completed with just a few simple clicks.
Try Our Custom SystemWhy Choose Us?
Discover the key advantages of our platform and read testimonials from our successful partnerships.
One-Stop Solution
Our integrated platform covers the entire CAR-T development pipeline, from initial discovery to preclinical validation, ensuring seamless project management.
Scientific Expertise
Our team comprises industry veterans with extensive experience in immunology and oncology, providing expert guidance and problem-solving.
Advanced Platforms
Leverage our cutting-edge technologies, including Smart™ CAR, mRNA, and CRISPR platforms, to build next-generation cell therapies.
Quality & Compliance
We offer GLP-compliant assays and adhere to the highest quality standards, providing reliable data packages ready for IND submission.
Trusted By
Frequently Asked Questions
Your questions, answered. Find information about our processes and services.
What starting materials do I need to provide?
To initiate a CAR-T development project, you typically need to provide the sequence of your binder (e.g., scFv) and the target antigen information. If you have a specific CAR construct design in mind, please provide those details as well. If you are at an earlier stage, we can start with just the target antigen, and our team can assist with binder discovery and CAR design as part of the one-stop service.
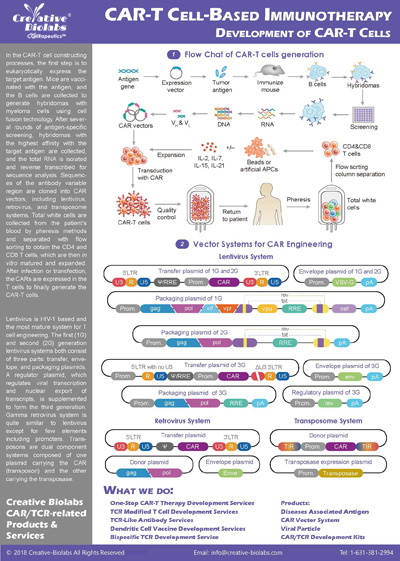
What viral vectors do you use for gene delivery?
Our standard and most recommended platform utilizes high-titer lentiviral vectors for stable and efficient transduction of T cells. We also have extensive experience with retroviral vectors. We can help you choose the best vector system based on your specific CAR construct, T cell source, and downstream application requirements.
What is the source of the T cells you use for CAR-T production? Can we provide our own cell samples?
We offer flexibility to meet your specific needs. We can source high-quality T cells from fully-vetted healthy donor peripheral blood mononuclear cells (PBMCs). Additionally, we are fully equipped and experienced in working with cells provided by our clients, including cryopreserved patient-derived samples for both autologous and allogeneic therapies.
Can you perform assays under GLP-compliant conditions?
Yes. We offer key preclinical assays, particularly in vivo toxicology and biodistribution studies, under Good Laboratory Practice (GLP)-compliant conditions to support your IND filing. Please specify your regulatory requirements during our initial discussion so we can ensure the study design and execution meet the necessary standards.
How do you ensure the confidentiality of my project?
We treat all client information with the strictest confidentiality. All projects are initiated under a mutual Confidentiality Agreement (CDA). Our data management systems are secure, and access is tightly controlled. Your proprietary sequences, data, and project strategy are safe with us.
Related Resources
Dive into our latest webinars, podcasts, and expert articles designed to provide valuable insights for your CAR-T program.
Videos
Infographics
Flyers
Support Knowledge
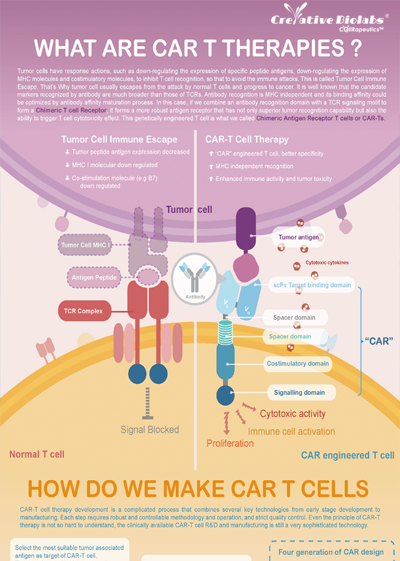
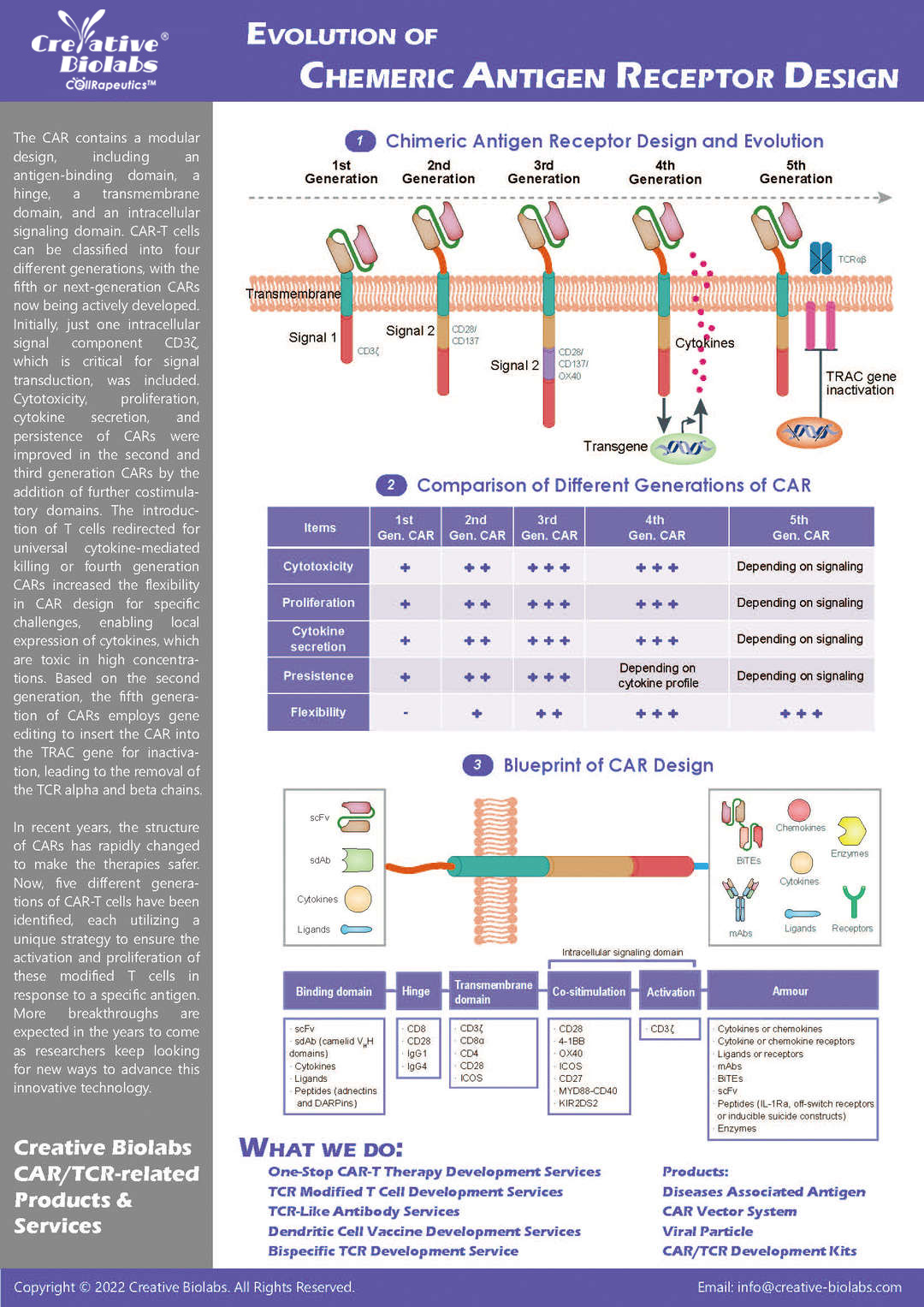
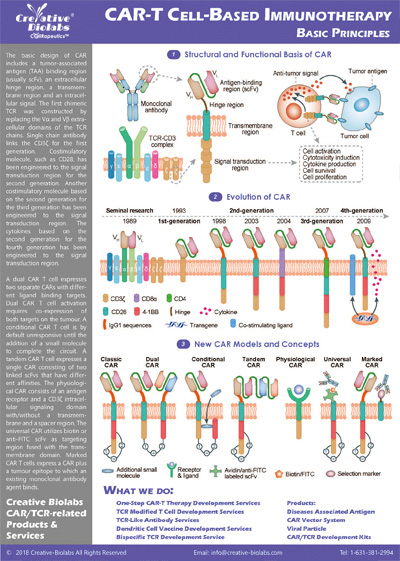
Overview of Chimeric Antigen Receptors
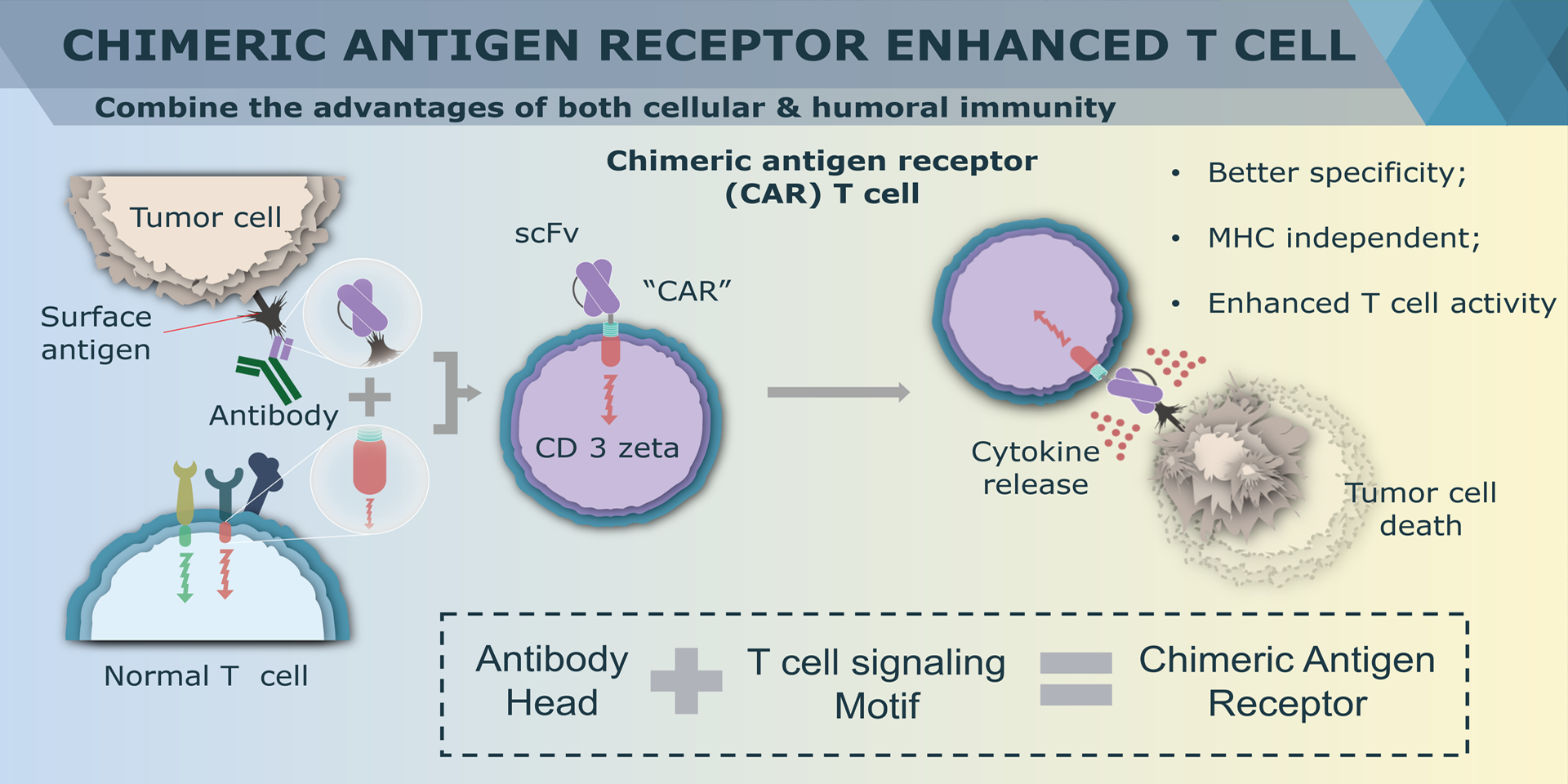
Chimeric antigen receptor (CAR) can combine the extracellular antigen recognition domain from antibodies with the immune cell signaling domain to redirect T cell specificity and induce potent antitumor activity. CAR is an artificial transmembrane receptor that connects the extracellular antigen recognition domain, hinge domain (HD), transmembrane domain (TMD), and intracellular signal transduction domain in series.
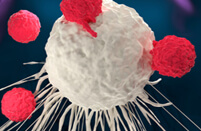
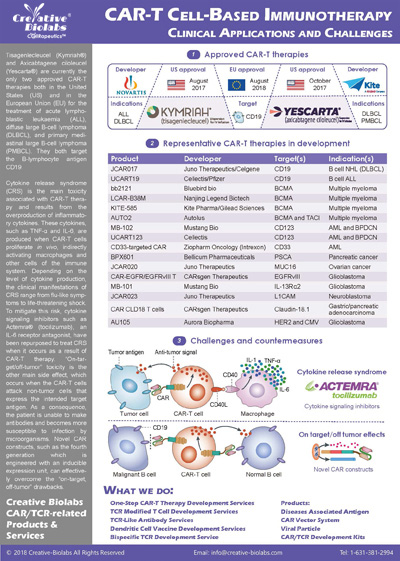
Overview of CAR-T Cell Therapies
CAR-T cell therapies revolutionize cancer treatment by harnessing the power of a patient's immune cells. Engineered with chimeric antigen receptors (CARs), these cells effectively target and destroy cancer cells, offering a personalized and potent approach. This groundbreaking immunotherapy has shown remarkable success in treating certain blood cancers, providing hope for patients who may not respond to traditional treatments. CAR-T cell therapies mark a significant stride towards precision medicine, ushering in a new era in oncology with the potential to transform the landscape of cancer care.
T Cell-based Immunotherapies
Based on the significant roles of T cells in the immune system, many small-molecule drugs targeting T cells and T-cell based immunotherapies have been developed for the treatment of intractable diseases including autoimmune diseases and cancer. T cell-based immunotherapies mainly utilize the mechanisms of T cell-mediated immune responses and the effects of some other immune cells such as dendritic cells (DCs), natural killer (NK) cells, and macrophages.
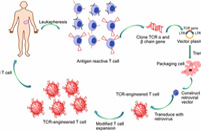
Adoptive Transfer of Engineered T Cells
Adoptive cell transfer (ACT) of engineered T cells is a cutting-edge therapeutic approach revolutionizing cancer treatment. This innovative method involves modifying T cells, a key component of the immune system, to enhance their ability to target and eliminate cancer cells. By introducing genetically engineered T cells into patients, researchers aim to bolster the immune response against cancer, offering a personalized and potentially curative treatment option. This groundbreaking technology holds promise for addressing various malignancies and represents a significant stride towards more effective and precise cancer therapies.