The use of modified chimeric antigen receptor (CAR) T cells (CAR-T) in immunotherapy has significantly improved the survival rates of pediatric patients with relapsed leukemia. However, these therapies are not as effective in treating solid tumors and may carry substantial toxicity. In a new study, researchers from St. Jude Children’s Research Hospital in the United States found that incorporating a modular chimeric cytokine receptor into CAR-T cells enhances their efficacy in various solid tumor models. The research findings were published online in the Nature Biomedical Engineering journal on November 30, 2023, under the title “Modular chimeric cytokine receptors with leucine zippers enhance the antitumour activity of CAR T cells via JAK/STAT signalling.”
The lead author of the paper, Matthew Bell, stated, “We designed modular chimeric cytokine receptors and demonstrated their ability to improve CAR-T cells. From our models, it appears that this technique has the potential to broadly enhance the effectiveness of CAR-T cell therapy for solid tumors and brain tumors.”
CAR-T cells are modified immune cells from the patient that can target and kill cancer cells. Signals from solid tumors can deactivate CAR-T cells, reducing the efficacy of treatment. To address this issue, scientists combined CAR-T cells with cytokine injections; however, this approach could lead to severe unexpected toxicity. The new method developed by the authors allows the delivery of pro-immune signals from cytokines exclusively to CAR-T cells, eliminating systemic toxicity.
Bell explained, “Our approach confines the action of cytokine signals solely to the modified CAR-T cells. In turn, this reduces the likelihood of cytokine-related toxicity and provides the signals necessary for these CAR-T cells to effectively function in the inhibitory tumor microenvironment.”
The corresponding author, Dr. Stephen Gottschalk from St. Jude Children’s Research Hospital’s Bone Marrow Transplantation and Cellular Therapy Division, said, “Our modular chimeric cytokine receptors represent an approach to improve current CAR-T cell therapy for solid tumors, as the effectiveness of this therapy has been somewhat disappointing in early clinical trials. Our preliminary study results suggest an improvement in tumor control in various model systems, which is promising.”
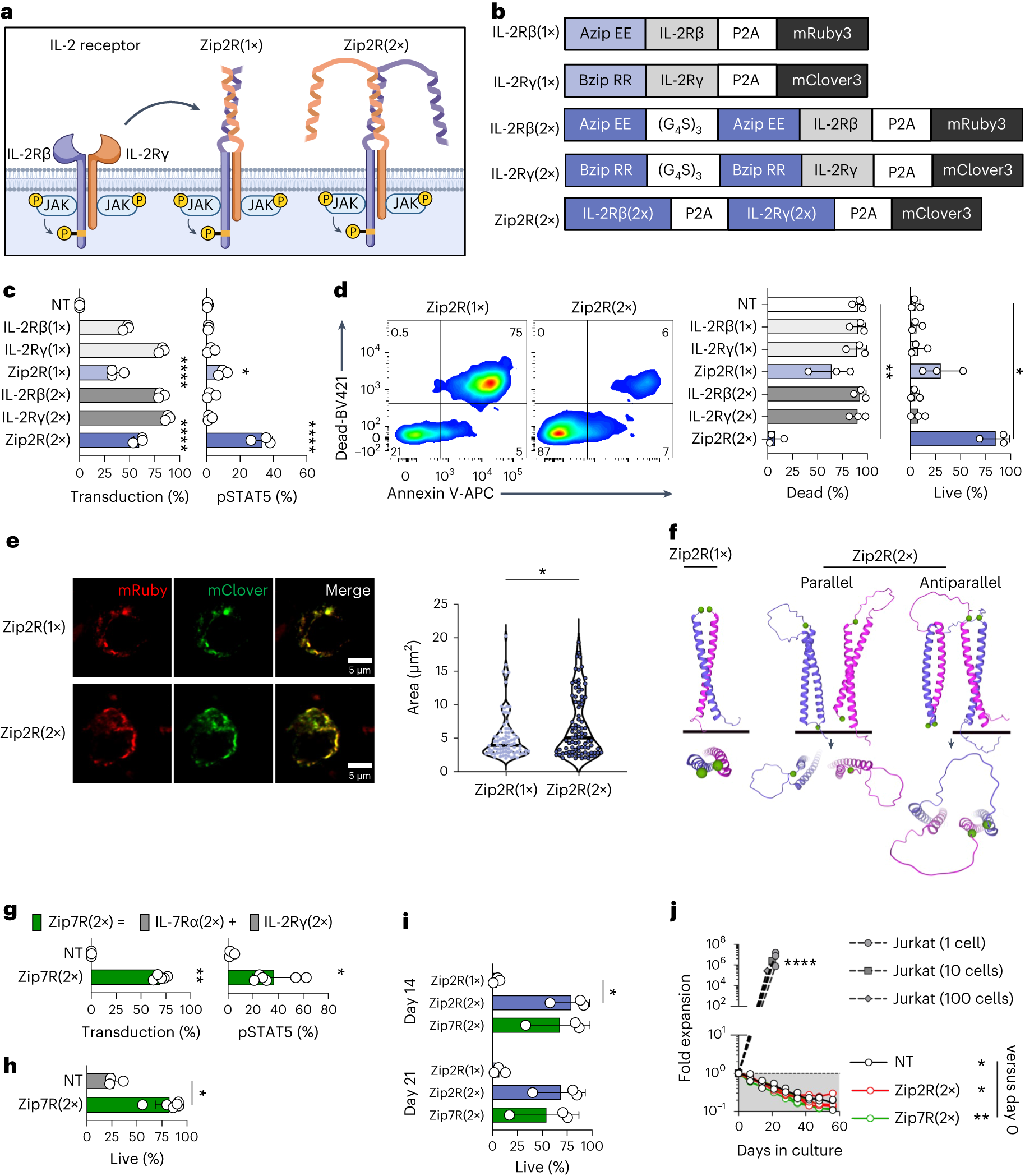
The authors replaced the extracellular domains of different cytokine receptors with a leucine zipper motif, creating constitutively active receptors. CAR-T cells expressing modular chimeric cytokine receptors demonstrated stronger anti-tumor activity against various cancer types in cell lines and mouse models compared to traditional CAR-T cells. While modular chimeric cytokine receptors provide a sustained “on” signal to CAR-T cells, they do not induce nonspecific proliferation of CAR-T cells.
Gottschalk explained, “We are pleased to find that modular chimeric cytokine receptors only slightly activate the cytokine pathway. In the absence of tumor cells, these receptors can enhance the survival of CAR-T cells without causing proliferation. To optimize the chimeric cytokine receptor design and characterization, we collaborated closely with several researchers from St. Jude Children’s Research Hospital, including Giedre Krenciute, Jiyang Yu, Junmin Peng, Hongbo Chi, and Madan Babu.”
Bell concluded, “Ultimately, we have identified a method to enhance CAR-T cell anti-tumor activity, which may be more effective and safer than cytokine injections.”
Reference
1. Bell, Matthew, et al. “Modular chimeric cytokine receptors with leucine zippers enhance the antitumour activity of CAR T cells via JAK/STAT signalling.” Nature Biomedical Engineering (2023): 1-17.