The detection and destruction of cancer cells by immune cells is the basis of all modern immunotherapies, including cancer vaccines, checkpoint blocking, and adoptive immune cell therapy (ACT). To achieve the production of a large number of tumor-specific T cells that ADC functionally depends on, two methods are used: 1) to isolate tumor-infiltrating lymphocytes (TILs) to produce T cell receptor (TCR) -modified T cells (TCR-T); 2) to transfer chimeric antigen receptor (CAR) into peripheral blood lymphocytes (PBLs) to produce CAR-T cells.
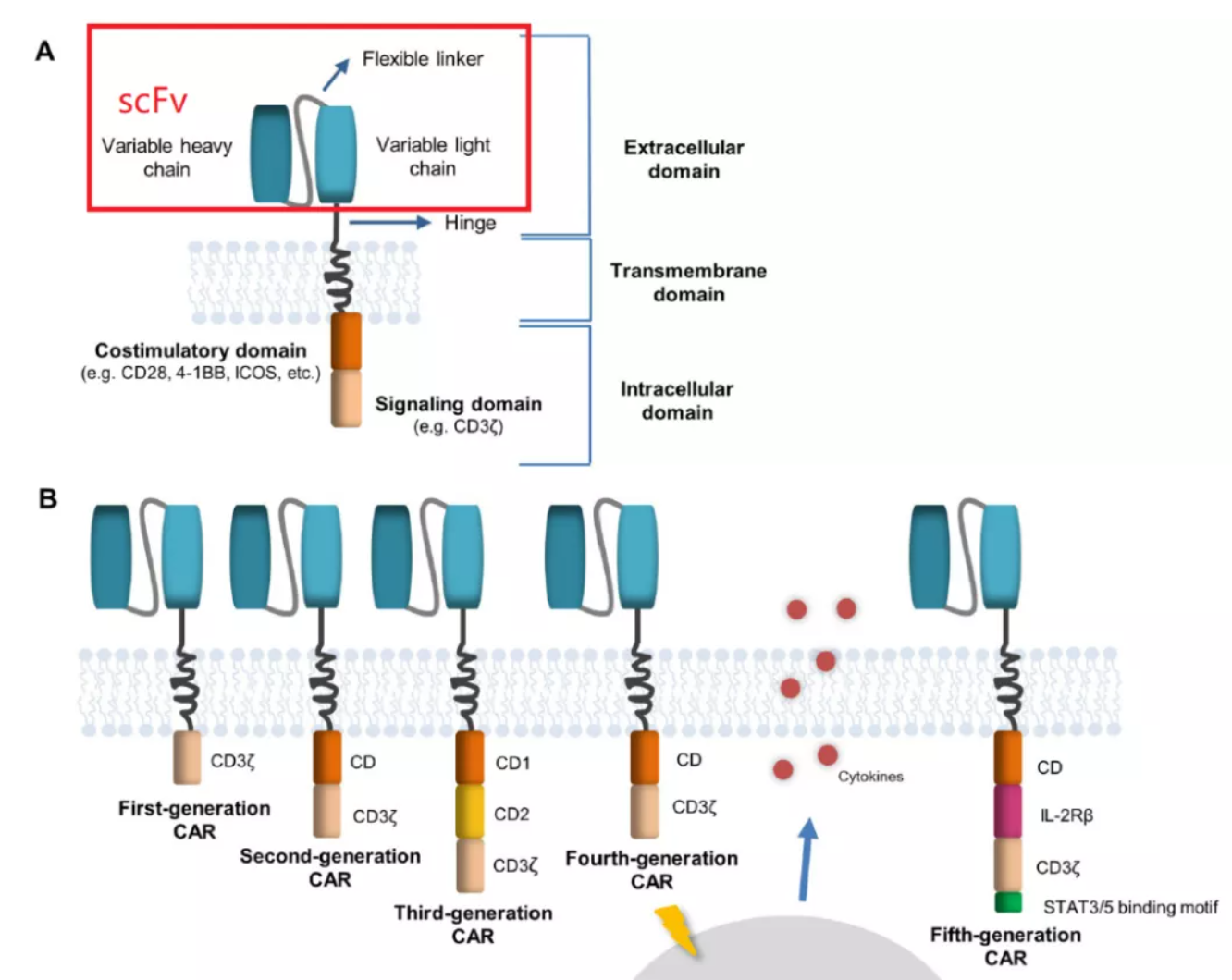
CAR-T cells combine the specificity of antibodies with the cytotoxicity of T cells, which is the main method of ACT at present. In the structural compositions of CAR, scFv determines the antigen specificity of CAR-T, and the antibody variable light chain (VL) is connected to the antibody variable heavy chain (VH) through a linker. The linker, affinity, MHC independence and immunogenicity of scFv affect the killing ability, persistence and on-target off-tumor toxicity of CAR-T.
scfv
The antigen-binding characteristics of CAR are determined by the antigen recognition domain, which is usually composed of a single-stranded variable fragment (scFv) formed by linking the variable light chain (VL) and variable heavy chain (VH) of the monoclonal antibody with a short linker. Theoretically, the sequence of VL-Linker-VH is closer to the design of natural antibodies. However, experimental tests have shown that both VL-linker-VH and VH-linker-VL can work normally in most cases.
Linker
Different linker molecules have been successfully used to design scFvs. At present, most of the linkers used in CAR-T cells include some variants based on glycine (Gly) and serine (Ser) repeat peptides. For example, (Gly4Ser)3 linker consists of three repeats of Gly-Gly-Gly-Gly-Ser. The purpose of using these residues is to enhance the linker’s flexibility to transform and minimize the interference to the function and folding of the connected protein domain. In addition, a linker should not be too short, or aggregates can be formed easily. Antigen-independent CAR aggregation will induce tonic signaling and affect the function of CAR-T. Therefore, the best linker length is 15-20 amino acids, usually using (Gly4Ser)3 or (Gly4Ser)4.
Affinity
One of the key determinants of CAR function is the affinity of scFv to its homologous antigens. The affinity of scFv-based CAR-T cells to their targets is generally several orders of magnitude higher than that of unmodified TCR-T cells. However, some molecules, such as the γ, δ and ε chains of CD4, CD8 and CD3, do not exist in CAR, which is beneficial to the activation of TCR- transmitted signals.
The optimal antigen affinity of CAR-T cells may vary with many factors, such as the design of costimulatory domain and spacer, the density of target cell antigen, and the expression of CAR in T cells. Studies have shown that the affinity of CAR has a downward limit, beyond which sufficient antigen recognition will not occur, resulting in unsatisfactory activation of CAR-T cells. However, for target cells with high antigen expression, increasing the affinity of CAR does not necessarily improve the function of CAR-T cells, meaning there is an upper limit of CAR affinity.
Fine-tuning the affinity of CAR can also reduce the binding to low-level antigens in normal tissues, reduce the toxicity of on-target off-tumor, and maintain sufficient effector function to eliminate malignant cells with over-expression of antigens.
MHC-independent activation
Another significant advantage of scFv-based CAR-T cells is that they can recognize target antigens independent of MHC antigen presentation, overcoming the tumor escape induced by the down regulation of MHC molecules, and enabling CAR-T cells to recognize non-peptide antigens, such as glycolipids or tumor-specific glycosylation.
The initial disadvantage of this MHC-independent CAR-T is that it can only recognize surface antigens. In recent years, antibodies to specific peptide-TCR complexes have been designed to mimic TCR recognition of presenting peptides, and the targets can be extended to a large number of intracellular proteins. The loss or down-regulation of the target antigen will inevitably lead to treatment failure, which can be resolved by designing multi-target CAR-T.
Target antigen
ScFv-based CAR-T cells can theoretically be redirected to any antigen. However, some scFvs may have unique characteristics, which limit their application in CART cells. The comparison between CAR of CD19(FMC63-scFv) and GD2(14g2a-scFv) found that the frame region of 14g2a-scFv induces the accumulation of anti-GD2 CAR on the surface of T cells, which leads to the activation of tonic CAR signal, and the rapid depletion of anti-GD2-CAR-T cells in vitro, which limits their normal function in vivo.
Immunogenicity
A problem in CAR-T awaits to be solved. Most clinical CAR-T trials use scFvs from mouse antibodies, which increases the immunogenicity of CAR-T and may lead to toxicity or limit the persistence of CART cells. This problem can be partially solved by humanized scFvs. However, due to the chimeric nature of these receptors, even structures derived entirely from human proteins may cause host immune responses. This reaction will be caused by the production of immunogenic peptide sequences at the connecting sites of the CAR domain or the induction of anti-idiotypic antibodies.
Reference
1. Abreu, Teresa R., et al. “Current challenges and emerging opportunities of CAR-T cell therapies.” Journal of Controlled Release 319 (2020): 246-261.