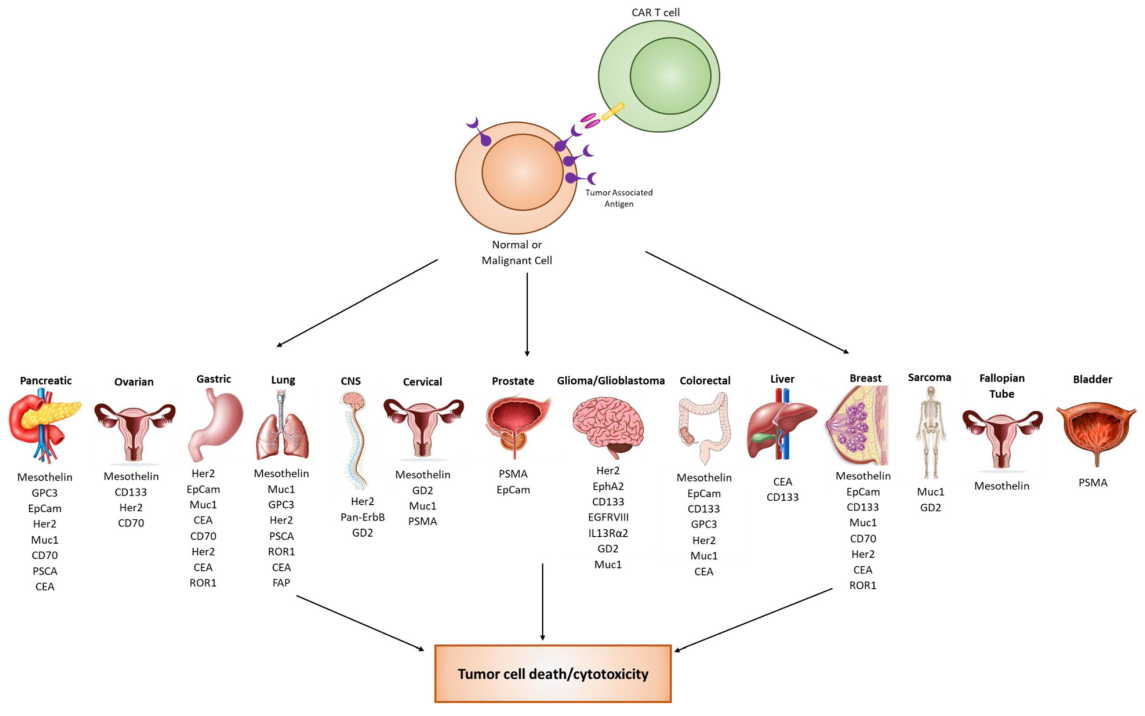
A recently published report summarizes the biomarker targets in both hematological and solid malignancies for CAR T cell therapy and discusses potential new biomarker target.
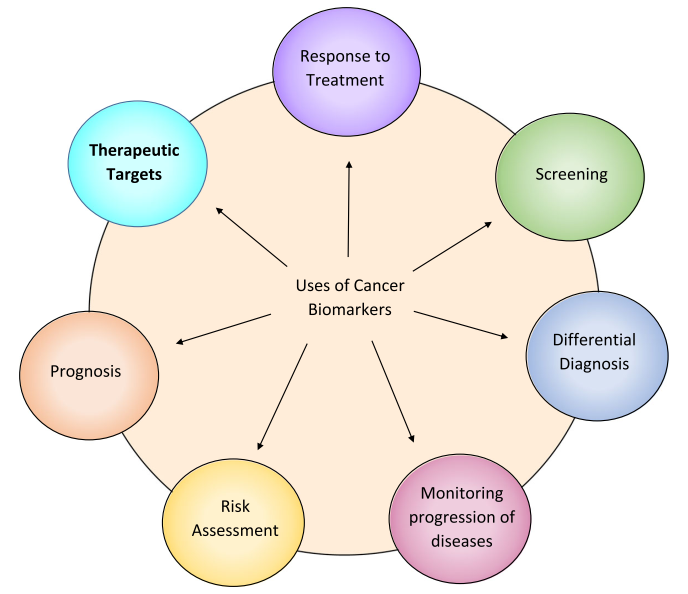
A biomarker is an integral part of cancer management due to its use in risk assessment, screening, differential diagnosis, prognosis, prediction of response to treatment, and monitoring the progress of disease (Figure1). Recently, with the advent of Chimeric Antigen Receptor (CAR) T cell therapy, a new category of targetable biomarkers has emerged. These biomarkers are associated with the surface of malignant cells and serve as targets for directing cytotoxic T cells.
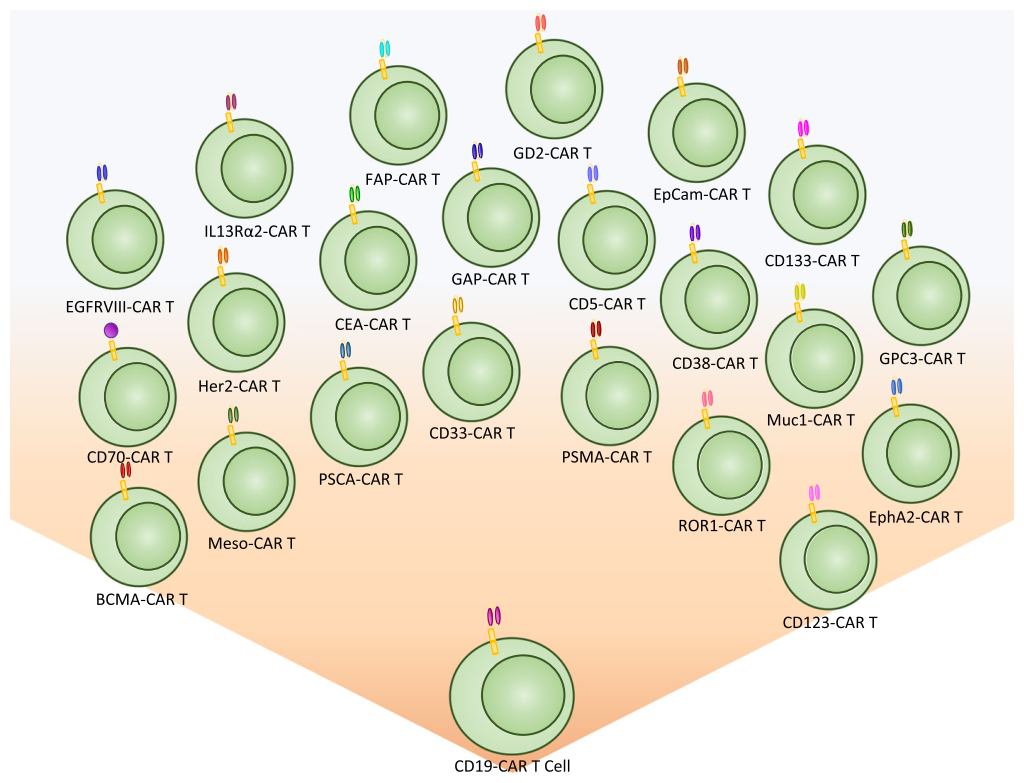
The first biomarker target used for CAR T cell therapy was CD19, a B cell marker expressed highly on malignant B cells. With the success of CD19, the last decade has shown an explosion of new targetable biomarkers on a range of human malignancies. These surface targets have made it possible to provide directed and specific therapy that reduces healthy tissue destruction and preserves the patient’s immune system during treatment.
1. Surface biomarkers have expanded significantly over the last decade
CAR T cell therapy was initially conceptualized in 1989 and was recognized as an effective therapeutic after targeting CD19 for the treatment of lymphomas and leukemias. This led to an exponential growth in CAR therapy and as a direct consequence, in surface biomarker discovery.
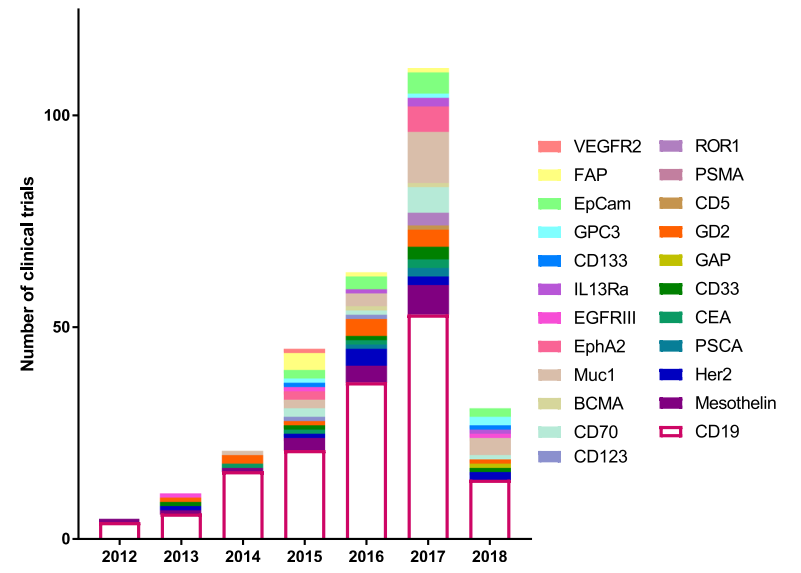
In 2012, there were a total of 5 clinical trials, four targeting CD19 and one targeting Mesothelin. This number has continued to grow and the number of biomarkers tested in a clinical setting has also expanded from 2 to 25. There were more clinical trials than any previous year in 2017 with 111 initiated, targeting 17 different biomarkers. This growth demonstrates not only the efficacy of CAR T therapy, but also the huge push in immunotherapy to find new and better targets.
2. Current clinical targets for hematological malignancies
As the most studied and researched target for CAR therapy, CD19 has shown impressive success in clinical settings to treat Acute Lymphoblastic Leukemia (ALL), Non-Hodgki Lymphoma (NHL), and Chronic Lymphocytic Leukemia (CLL).
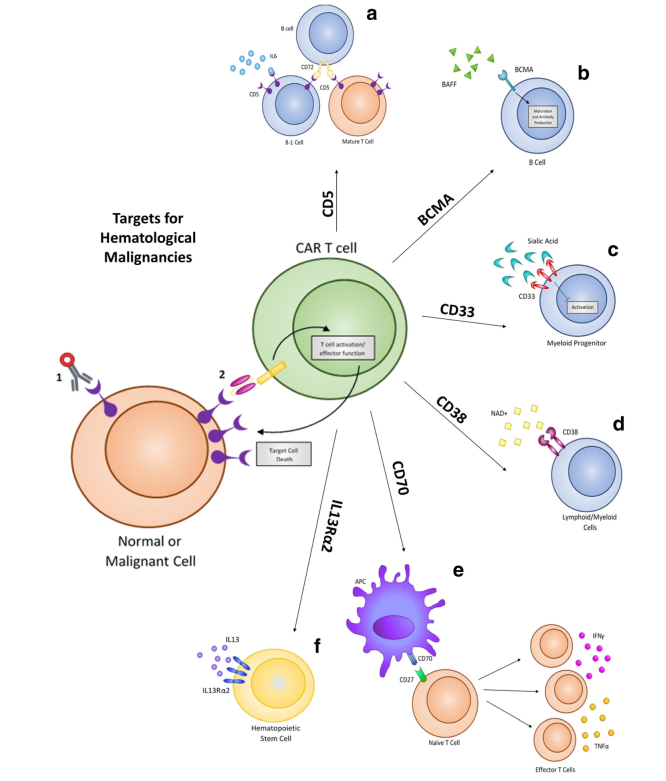
Despite the high levels of complete response rates in patients, relapse from CD19 CAR therapy can occur via a suppressive tumor microenvironment or antigen escape. Based on this, new targets are being identified and evaluated to treat hematological malignancies, including CD5, IL3Rα (CD123), CD33, CD70, CD38, and BCMA. These same targets have already shown promise using drug-conjugated antibodies, and several have been approved by FDA for treatment. These biomarkers are now being evaluated as targets for adoptive T cell CAR therapy to treat hematological malignancies.
3. Current clinical targets for solid tumors
While CAR T cell therapy has been very successful against hematological malignancies, it has been challenging to apply this technology to solid tumors. This challenge has resulted in a strong effort to discover biomarkers for solid malignancies. There are 17 biomarkers currently in clinical trials for solid tumors. Over 14 different organ types are currently being targeted using a variety of different biomarkers. Many biomarker targets are expressed in several different cancer types.
4. Upcoming biomarkers
As CAR therapy expands, so does the need for discovering new cancer-specific biomarkers that can serve as targets. There are some biomarkers with preliminary preclinical data that may be useful as future CAR targets.
- CT antigens
Cancer/testis (CT) antigens have normal expression limited to adult testicular germ cells, but have shown expression in various tumor cells such as ovarian cancer, lung cancer, melanoma, breast cancer, glioma, and colon cancer. Since male germ cells are unable to present antigens to T cells, CT antigens can be targeted with minimal cytotoxicity to normal tissue. While current efforts to target CT antigens are primarily focused on modified high specific TCR regions, there is an opportunity to target these antigens using CAR T cells as well.
- GUCY2C
Guanylyl cyclase C (GUCY2C) is a membrane-bound protein found on the apical surfaces of intestinal epithelial cells, but is also a cancer mucosa antigen overexpressed in both primary and metastatic colorectal cancers as well as esophageal and gastric cancers. It has been determined that CD8+ T cell responses are expanded when cells are vaccinated against GUCY2C. These cells are effective at eliminating metastatic colorectal tumors. Initial GUCY2C targeting with CAR T cells has shown promising specificity and demonstrated reduced tumor number and increased survival in mice with GUCY2C+ tumors. This target shows potential for the possible CAR T cell treatment of colorectal tumors in human patients.
- TAG-72
Tumor associated glycoprotein-72 (TAG-72) is a pancarcinoma antigen that shows expression in ovarian cancer, colorectal cancer, breast cancer, and prostate cancer. While TAG-72 is present in the normal female reproductive tract, the expression is limited and generally weaker than that seen in cancer. While 91% of endometrial adenocarcinoma samples showed TAG-72 expression, the expression of TAG-72 in normal tissue appears to be hormone (estrogen and progesterone) dependent, which can be utilized to prevent.
- HPRT1/TK1
Salvage enzymes Thymidine Kinase 1 (TK1) and Hypoxanthine guanine phosphoribosyl transferase (HPRT1) have recently shown potential as surface antigens for CAR T cell therapy. HPRT1 is a salvage pathway enzyme that synthesizes guanine and inosine throughout the cell cycle. The protein is a housekeeping protein found within all normal somatic cells in low levels. There is an upregulation of HPRT1 in certain cancer types, making it a promising biomarker for the treatment of these cancers. In addition, the protein has also been shown to have significant surface localization on certain malignancies such as lung and colorectal cancer. As HPRT1 expression is limited to the cytosol within normal cells, the unique surface localization of the protein makes it a promising targetable CAR T biomarker. TK1 is another salvage enzyme responsible for the synthesis of thymidine in the cell cycle and has been used as a serum biomarker for cancer detection and recurrence. Recent evidences show that TK1 may also be upregulated within some malignancies and displayed on the surface of the cell. As proteins normally restricted intracellularly, TK1 and HPRT can be used as surface antigens for CAR therapy with minimal bystander cytotoxicity.
5. Conclusion
The search for new biomarker targets for both hematological and solid malignancies with the expansion of CAR T cell therapy. Immunotherapy is becoming the new standard in patient care and has experienced huge growth and expansion over the last decade. As CAR T cells become more sophisticated and new biomarkers are discovered to expand treatment to numerous cancer types, the field of immunotherapy will cover more patients and aid in the improvement of care.
Reference
Townsend M H, Shrestha G, Robison R A, et al. The expansion of targetable biomarkers for CAR T cell therapy. (2018)