This is a review article published on Cell Research (2017) 27: 38-58 by Laura A Johnson and Carl H June. Dr. Carl H. June is the director of translational research at the Abramson Cancer Center at the University of Pennsylvania. He is most well-known for his research into T-cell therapies for treatment of cancer. The June Lab is primarily responsible for developing new CARs and new vectors for current and proposed indications. In 2012, Dr. Laura A Johnson moved to Philadelphia to work with Dr. Carl June at the University of Pennsylvania, where she specializes in translational research of gene-engineered T cell therapy of solid tumors. This work is currently in clinical trials to treat patients with glioblastoma with chimeric-antigen-receptor engineered T cells targeting the tumor-specific mutation, epidermal growth factor receptor variant three (EGFRvIII).
Abstract:
Chimeric antigen receptor (CAR) gene-engineered T cell therapy holds the potential to make a meaningful difference in the lives of patients with terminal cancers. For decades, cancer therapy was based on biophysical parameters, with surgical resection to debulk, followed by radiation and chemotherapy to target the rapidly growing tumor cells, while mostly sparing quiescent normal tissues. One breakthrough occurred with allogeneic bone-marrow transplant for patients with leukemia, which provided a sometimes curative therapy. The field of adoptive cell therapy for solid tumors was established with the discovery that tumor-infiltrating lymphocytes could be expanded and used to treat and even cure patients with metastatic melanoma. Tumor-specific T-cell receptors (TCRs) were identified and engineered into patient peripheral blood lymphocytes, which were also found to treat tumors. However, these were limited by patient HLA-restriction. Close behind came generation of CAR, combining the exquisite recognition of an antibody with the effector function of a T cell. The advent of CD19-targeted CARs for treating patients with multiple forms of advanced B-cell malignancies met with great success, with up to 95% response rates. Applying CAR treatment
to solid tumors, however, has just begun, but already certain factors have been made clear: the tumor target is of utmost importance for clinicians to do no harm; and solid tumors respond differently to CAR therapy compared with hematologic ones. Here we review the state of clinical gene-engineered T cell immunotherapy, its successes, challenges, and future.
Keywords: chimeric antigen receptor; T cell immunotherapy; CD19
This review starts from the firs concept of T-cell therapy of cancer — published report out of Steven Rosenberg’s group at the National Cancer Institute (NCI) Surgery Branch used patient T cells extracted from tumor (or tumor-infiltrating lymphocytes, TIL), expanded ex vivo and re-infused to successfully treat metastatic melanoma. Then discusses the development of TCR-engineered lymphocytes and chimeric antigen receptor t cell technology. Particularly, from the perspective of clinical investigation, the author lists current TCR clinical trials and solid tumor CAR-T clinical trials and concludes the target tumor markers used in completed and ongoing TCR and CAR gene-engineered T-cell immunotherapy clinical trials in a figure.
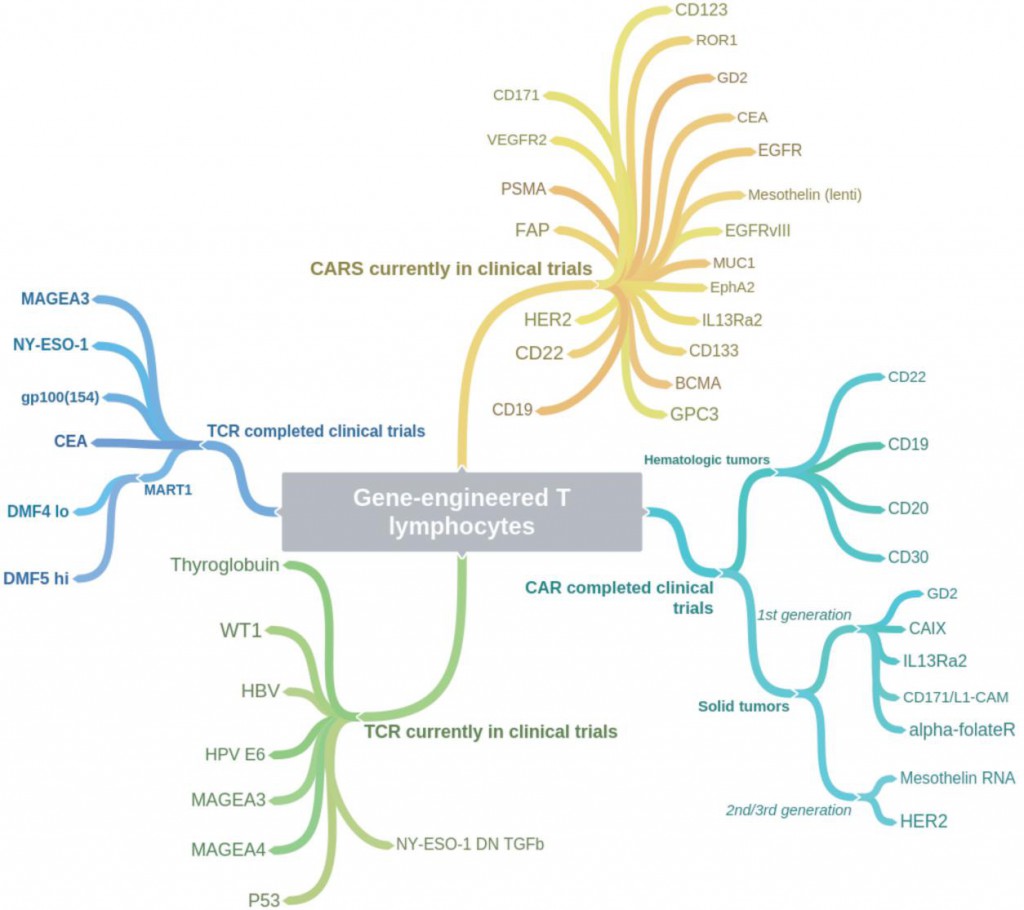
Figure 1. Coggle diagram of completed and ongoing TCR and CAR gene-engineered T-cell immunotherapy clinical trials (Johnson, 2017)
In the early age of T cell therapy research, scientists focused on the research of tumor-infiltrating lymphocytes and achieve some progresses. Though exciting but this approach proved to have its own challenges. Not all patients have resectable tumor, of those with resectable disease, not all tumors grew lymphocytes; of those that grew lymphocytes, not all demonstrated anti- tumor activity; of those that demonstrated anti-tumor function, many patients would not survive the eight-plus weeks required to grow and expand their T cells to therapeutic levels for reinfusion. This quandary brought about the observed need for a ‘universal’ T cell that could recognize tumors in different patients.
Based on the theory that T cells targeting shared antigens could be used to target similar tumors from different patients sharing the same HLA, scientists began to use TCR engineered T cell to treat patients. Patient peripheral blood T cells were transduced with a retroviral construct expressing the T-cell receptor (TCR) of certain antigen, and reinfused back to the patient intravenously. While TCR gene-engineered T-cell therapy has certainly proven to be potent, and efficacious in some cases, it faces a number of caveats. First and foremost is that it is necessarily restricted to a subset of patients. Not only do patients’ tumors have to express the protein of interest, they must also match the haplotype restriction of the TCR, and both the HLA molecule and the intracellularly processed peptide must be presented together on the surface of the tumor cell. By necessity, this limits the therapy to a small subset of patients.
CARs were originally conceptualized by Zelig Eshhar and colleagues in 1989, they described the generation of an “Immunoglobulin-T-cell receptor chimeric molecule” by splicing the heavy and light chain variable regions of a monoclonal antibody (mAb) followed by transfection of these two fragments along with the constant region of a TCR into a T lymphocyte cell line. They later modified their approach by generating a single-chain fragment (scFv) encoding both heavy and light variable regions joined by a linker sequence, negating the need for multiple gene transfers to achieve antibody-like receptor specificity.
Part of the problem in applying gene-engineered T cells to clinical treatment of patients with cancer, is that the field is so new that researchers and clinicians are still learning the biology of the system, and the ‘rules’. Choice of target alone may not be sufficient to predict toxicity or efficacy; additional attributes include method of gene delivery into T cells to sustain transgene expression (DNA/RNA/plasmid, gamma retrovirus, lentivirus, non-viral transposon recombination), method of stimulating (mAbs versus beads versus cells versus antigen) and growing T cells ex vivo (cytokine mixtures, duration of culture, and bulk versus selected cells), and T cell subsets (naïve versus effector versus memory). All of these minor differences can impact T cell engraftment, survival, and function in patients. The length of construct, choice of hinge region, location of target epitope, all contribute and differ in each case. Additionally, the choice of costimulatory molecule is a contentious issue. Even without the differences already noted, it appears that targeting the exact same antigen may have different results, depending on either the antigenic epitope targeted, or the affinity of the mAb scFv.
100 different types of solid tumor cancers seem to be more like 100 different diseases each with their own barriers to treatment. Likely a return to basic science will be required to elucidate the suppressive barriers encountered in each. While no one can predict exactly where the research will lead, one thing is certain, the future of cancer immunotherapy is bright indeed.
If you have more interests about CAR-T clinical trial and corresponding biomarkers, please visit our website and get the Global CAR-T clinical trial review PDF file for detailed information.
Reference
Johnson, Laura A., and Carl H. June. “Driving gene-engineered T cell immunotherapy of cancer.” Cell research 27.1 (2017): 38-58.
