Complement factor B (CFB) inhibitors exert therapeutic effects by specifically binding to factor B, inhibiting abnormal activation of the complement bypass pathway, and controlling intravascular and extravascular hemolysis in paroxysmal sleep hemoglobinuria (PNH).
Key Role of Alternative Pathways in the Pathologic Process of PNH
The complement system is an important component of intrinsic immunity and is activated to be physiologically active, while abnormal activation can lead to disease. There are three pathways to activate the complement system: the classical, the lectin, and the alternative. The three pathways have different front-end reactions, but the end pathway is the same, which is through the cleavage product of C5, C5b, and other complement proteins, C6, C7, C8, C9, to bind to the membrane-attacking complex (MAC), attacking the cell membrane to form a hydrophilic pore, which allows a large number of soluble small molecules, ions, and water molecules to freely pass through the cell membrane, resulting in the rupture of the cell.
Based on the activation mechanism of the complement system, several pharmaceutical companies have developed several C5 inhibitors to treat diseases caused by abnormal activation of the complement system by directly blocking the terminal pathway. Although C5 antibodies are internationally recognized as the standard therapy for PNH, many patients still suffer from anemia and other symptoms after receiving this therapy, and the emergence of new mechanism drugs is urgently needed in the clinic.
As research progressed, the critical role of the alternative pathway in the pathologic process of PNH was revealed.
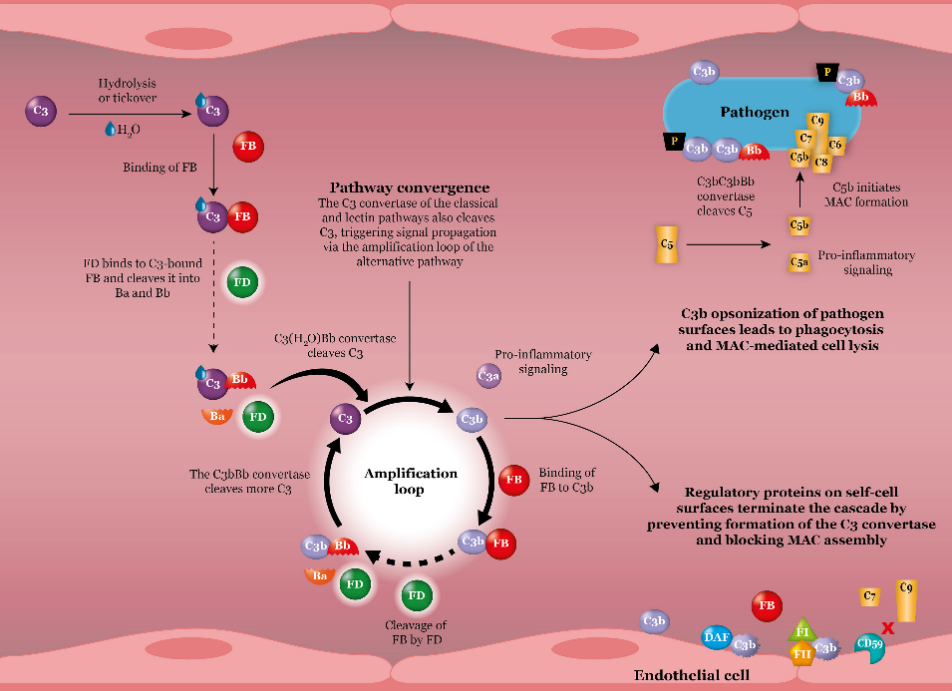
Fig. 1 Alternative pathways.1
In the alternative pathway, the natural cleavage product of C3, C3b, binds to factor B and then is cleaved by factor D, generating C3 convertase, which can further cleave C3 to produce C3b, which in turn can continue to generate C3 convertase under the action of factors B and D. The cycle continues in this way, forming an amplification loop that rapidly amplifies complement signaling. The amplification loop produces a large number of C3b and C3 converting enzymes binding to form C5 convertase, the enzyme can further cleave C5 to produce C5b, C5b, and other complement proteins binding to form a large number of MAC, and ultimately lead to the rupture of erythrocytes in the blood vessels, the occurrence of intravascular hemolysis.
Why are C5 Inhibitors so Ineffective?
There are two main reasons.
First, the large amount of C3b produced by the amplification loop can form a “C3b-rich” C5 convertase, which has a higher affinity for C5 and competes with C5 inhibitors for C5 binding, thus continuing to play a role in C5 cleavage and ultimately leading to intravascular hemolysis.
Second, C3b can sustain deposition on the erythrocyte surface, inducing erythrocyte destruction by the liver and spleen and extravascular hemolysis. Targeting complement factor B, selectively inhibiting the bypass pathway of the complement system, and blocking the amplification loop to amplify complement signaling are expected to play a better therapeutic role.
Development of Small Molecule Inhibitors Targeting Complement Factor B
Based on the existing knowledge, pharmaceutical companies actively lay the development of small molecule inhibitors targeting complement factor B. Initially, developers designed three high-throughput screening methods for lead compound discovery, including:
- Compound screening based on ELISA assay to measure MAC formation
- Compound screening based on the cleavage of C3 by CVF-Bb complexes
- Fragment screening based on NMR and X-ray
All three methods yielded more interesting lead compounds, and compound 1 obtained by the second screening method was finally chosen for further study. The developers quickly cultivated a co-crystalline complex of compound 1 with the catalytic domain of human complement factor B (hCFB) and performed structural analysis.
Before structural optimization, the developers performed off-target effect assessment and structural modification based on the assessment results. Finally, the compounds with optimal drug-forming properties were obtained. To further support the mode of action of the compounds with the ligand binding domain, the researchers cultivated and structurally analyzed the eutectic complexes of the final compounds with hCFB, and the binding modes were consistent with the assumptions.
Throughout drug discovery, from the initial in-depth understanding of the disease mechanism to the selection of target identification to high-throughput screening to discover lead compounds, and then through the use of the eutectic structure of the structure-based drug design, and ultimately discover the “first in class” drugs. The whole process is interlocked and logical, which is worth studying and learning from.
Reference:
- Barratt, J., and I. Weitz. “Complement factor D as a strategic target for regulating the alternative complement pathway. Immunol.12, 712572.” 2021. Distributed under Open Access license CC BY 4.0, without modification.
