Creative Biolabs is proud to offer a range of complement component inhibitors development services in a timely and cost-effective manner. With years of ample experiences in drug development, our scientists are confident in providing a full range of component inhibitors development services to promote the development of biopharmaceuticals. We offer turn-key or ala carte services customized to our client’s needs.
The complement system is an essential component of innate immunity, bridging the innate immune response to the adaptive immune response. But inappropriate activation or regulation of complement cascade may turn the destructive capabilities to host cells and is involved in lots of diseases and pathological conditions. Modulation or Inhibition of complement activity has therefore been regarded as a promising diagnostic or therapeutic strategy for a lot of diseases.
Although antibodies might be considered the useful archetype of site-blocking compounds, smaller molecules such as synthetic molecules, nucleotides, and peptides may also have the possibilities to interrupt protein functions by the induction of conformational changes or steric hindrance. Besides, small functional inhibitors of complement activity are recognized to have drug-like properties with enhanced pharmacokinetic profiles. These advantages may make them suitable for drug development efforts related to oral bioavailability and better administration routes.
Compstatin
Compstatin is the most well-developed candidate in this class of substances and entered clinical trials recently. This cyclic tridecapeptide has been disclosed through screening of the combinatorial phage-display libraries. It prevents the cleavage of C3 to its active fragments C3a and C3b effectively and therefore inhibits the most essential step in complement cascade. Although the exact mechanism of compstatin’s inhibitory action hasn’t been resolved, a recent publication suggests that the disruption of protein-protein interactions during convertase formation can be an important determinant. This finding has caused much speculation that a conformational change or destruction in protein-protein interactions is responsible for its activity. Compstatin has shown effective complement inhibition in a variety of experimental disease models. Despite a narrow selectivity for primate C3 that may affect preclinical animal experiments, its high efficacy and small size make it a promising drug candidate. Its inhibitory efficacy has been increased by 264-fold, through the use of rational and combinatorial synthesis, structural analysis, and computational approaches to identify active analogs.
Combinatorial Inhibitors
Combinatorial approaches have also been studied to identify a short aptamer molecule directed against C5. Aptamers have molecular recognition properties, which are single-stranded nucleotide stretches. And they offer better options for derivatization than antibodies. They can be chosen in an automated high-throughput process named systematic evolution of ligands by exponential enrichment (SELEX). A PEG-ylated anti-vascular endothelial growth factor (VEGF) aptamer for the therapy of wet age-related macular degeneration, was approved by FDA. The optimized and greatly modified form of the anti-C5 RNA aptamer features a subnanomolar binding affinity for human C5. This approach provides complete blockage of downstream complement activation by inhibiting the cleavage of C5 into C5a and C5b. A 40-kDa PEG group has been added to the 5' end for its pharmacokinetic properties improvement.
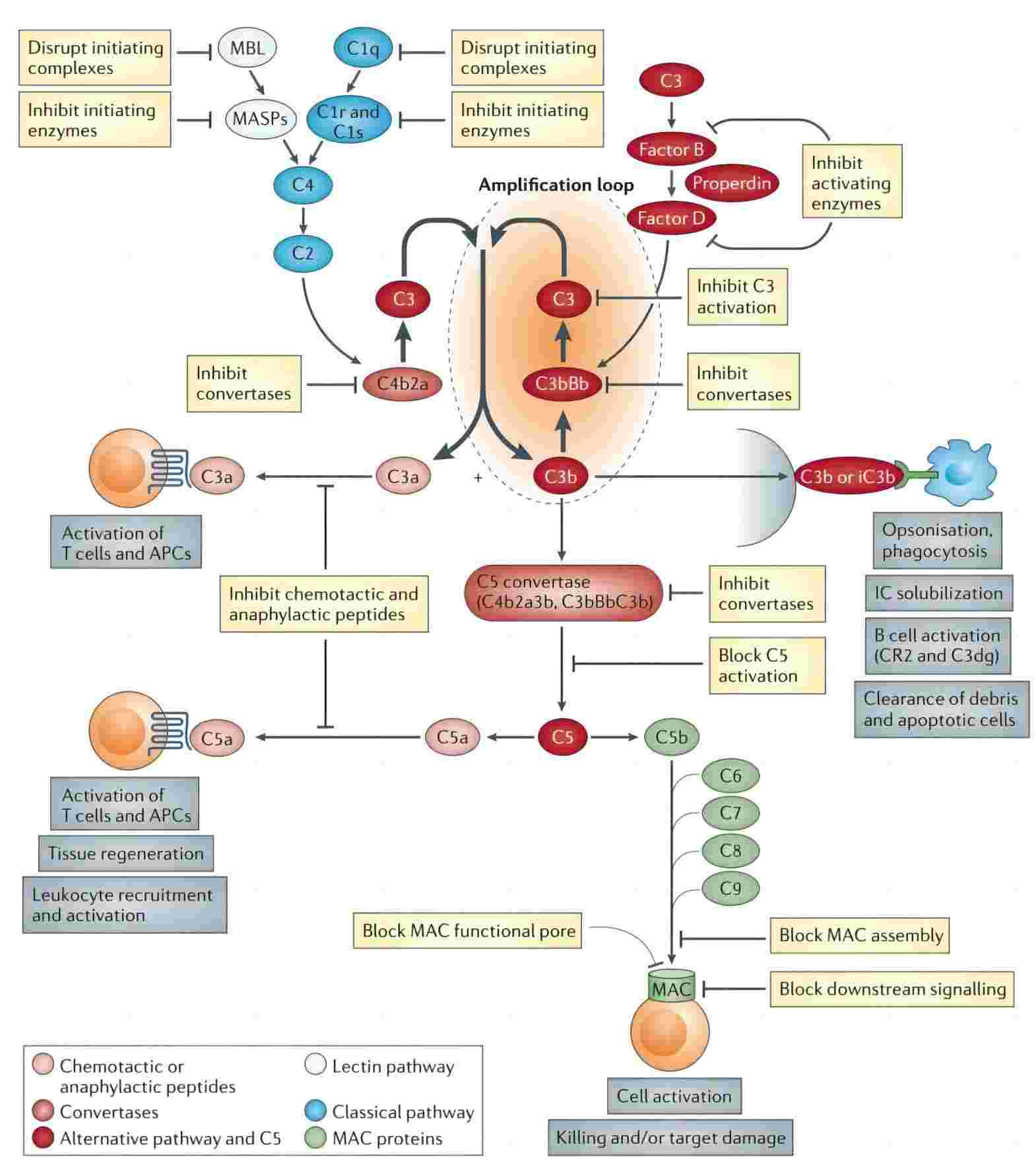
Fig. 1 Inhibition of the complement system.1
Based on our advanced complement component inhibitors strategy and well-developed drug discovery platforms, Creative Biolabs is confident in proving customized inhibitors against a variety of complement component, especially for those which are essential for complement activities. Please contact us for more information or a detailed quotation.
Reference
1. Morgan, B. Paul, and Claire L. Harris. "Complement, a target for therapy in inflammatory and degenerative diseases." Nature reviews Drug discovery 14.12 (2015): 857-877.
Related Product
For Research Use Only.
Related Sections:

